Grand Rounds
When Germline Genetic Testing Results Are Unclear: Highlighting Variants of Uncertain Significance
Patricia Kelly,(1) DNP, APRN, CNS, AGN-BC, AOCN®, Suzanne Mahon,(2) DNS, RN, AOCN®, AGN-BC, FAAN, and Patricia Friend,(3) PhD, APRN-CNS, AOCNS®, AGN-BC
From (1)Genomics Consultant, Dallas, Texas; (2)Saint Louis University, St. Louis, Missouri; (3)Marcella Niehoff School of Nursing, Loyola University Chicago, Illinois
Authors’ disclosures of conflicts of interest are found at the end of this article.
Correspondence to: Patricia Kelly, DNP, APRN, CNS, AGN-BC, AOCN®
E-mail: pakelly1027@sbcglobal.net
J Adv Pract Oncol 2023;14(7):631–638 |
https://doi.org/10.6004/jadpro.2023.14.7.7 |
© 2023 BroadcastMed LLC

 ABSTRACT
ABSTRACT
With the advent of high-throughput next-generation sequencing (NGS) and multigene panel testing, genetic testing and interpretations have become increasingly complex. Specifically, reports demonstrating “variant of uncertain significance” (VUS) present interpretative challenges. Misinterpretation of a VUS may result in clinical harm, emotional distress for patients and family members, and potential health-care provider liability. The following article and deidentified case study illustrate how a lack of health-care provider and patient understanding of a germline VUS resulted in a negative patient outcome and unnecessary surgery.
ARTICLE
Case Study
LP is a 40-year-old premenopausal woman who was seen by a nurse practitioner for her well-woman exam. Because of her personal and family history of colon polyps, LP expressed interest in genetic testing to assess for hereditary cancer risk. LP’s health-care provider took a “shotgun” approach and ordered a multicancer 81-gene panel that included genes associated with an increased risk of developing cancer in multiple major organs, including breast, gastrointestinal, endocrine, prostate, and gynecologic systems. LP does not recall receiving information on the risks and benefits of genetic testing nor the potential for a variant of uncertain significance (VUS). For LP, it was a “routine blood test.” The germline genetic testing results demonstrated a reportable finding: a “variant of unknown clinical significance” in the PALB2 gene.
LP’s health-care provider interpreted the report to mean that the patient was at significant risk for PALB2-associated cancers and arranged for LP to have a hysterectomy and bilateral oophorectomy (HBSO) to reduce the risk for ovarian cancer. Following the HBSO, LP was referred to a breast surgeon for consideration of risk-reducing
bilateral mastectomies. Upon review of the genetic testing results from the 81-gene panel, the breast surgeon determined that the VUS result needed further clarification before additional surgery and referred the patient to a genetics advanced practice registered nurse (APRN) with advanced education and genetics credentialing. (From this point forward, the APRN will be referred to as G-APRN.)
It should be noted that genetic testing for hereditary cancer susceptibility may also be referred to as “germline” testing or “germline biomarker” testing. Germline indicates an inherited genetic alteration in the DNA that is present in a body’s reproductive cells (egg or sperm) and is incorporated in the DNA of all cells (National Cancer Institute [NCI], n.d.). Biomarker is defined as a biological molecule found in blood, other body fluids, or tissues that is a sign of a normal or abnormal process, or of a condition or disease (NCI, n.d.). One of the germline genetic testing goals is to identify individuals who are at risk for cancer and would benefit from early detection or risk-reducing surgeries (The Jackson Laboratory, n.d.).
Health History
LP’s medical history was unremarkable except for three colon polyps that were removed at age 35 during a colonoscopy. The colonoscopy was done because of a family history of colon polyps. LP was told that the polyps were adenomatous, and she should return in 5 years for a follow-up colonoscopy. The G-APRN reviewed the polyp pathology report and confirmed the polyps were adenomatous without dysplastic or high-risk features. LP had not had a mammogram and had no history of abnormal breast examinations or breast biopsies. Except for the recent HBSO, LP had no other surgical procedures nor was she taking any medication. LP reported vaginal dryness and hot flashes post HBSO.
Family History
The G-APRN constructed a three-generation pedigree (Figure 1). LP’s father and sister had colon polyps at ages 45 and 30, respectively (pathology reports not available). LP’s family history included a father with a smoking history who died of lung cancer at age 59, sister with thyroid cancer (age of onset and pathology unknown), paternal aunt with breast cancer (age of onset unknown), a paternal uncle with prostate cancer (age of onset unknown), and a paternal grandmother who died of lung cancer (age of onset unknown). The maternal and paternal lineage was of western European descent, with no known Ashkenazi Jewish heritage. Besides LP, no other family members had undergone genetic testing.
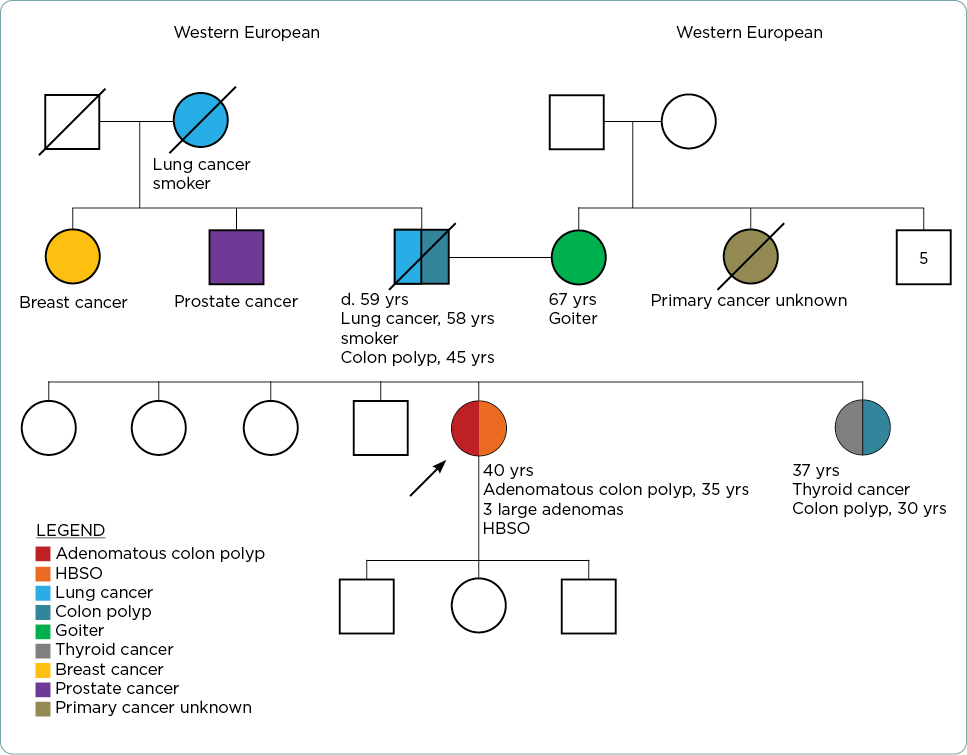
Genetic Testing Report
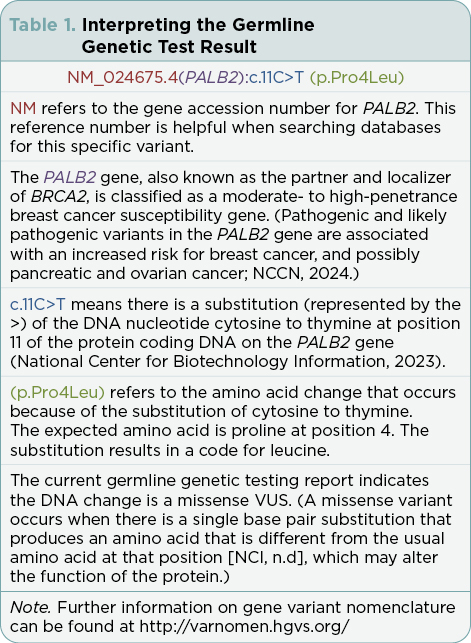
The G-APRN reviewed the genetic testing report that indicated a PALB2 variant: NM_024675.4(PALB2):c.11C>T (p.Pro4Leu) (Table 1). According to the laboratory report, the results were considered to be a VUS. The G-APRN consulted ClinVar, a freely accessible public archive of human genomic variation (National Library of Medicine, n.d.). The G-APRN noted this variant, (PALB2):c.11C>T (p.Pro4Leu), is listed as “uncertain significance” based upon seven submissions and “likely benign” based upon four submissions, as of November 4, 2023 (National Center for Biotechnology Information, 2023).
Assessment
LP does not have a personal or family history of colon cancer. LP has fewer than 10 polyps, the threshold for considering genetic testing for a hereditary colon cancer syndrome (National Comprehensive Cancer Network [NCCN], 2023). Her family history of colon polyps is unclear. More information is needed from her sister and father about the number and type of colon polyps identified. The G-APRN asked LP to obtain information (age of cancer onset/pathology reports) for her sister, paternal aunt, uncle, and grandmother. Further genetic testing is not indicated at this time.
Plan
- Provide education about VUS based upon present knowledge of the (PALB2):c.11C>T (p.Pro4Leu) variant. Emphasize that at this time, the variant is not medically actionable and risk-reducing mastectomies are not indicated. Based upon current information, testing other family members for the PALB2 VUS is not indicated.
- Assess LP’s distress level and reaffirm reasons for requesting genetic testing.
- Instruct LP to check with a G-APRN professional and the genetic testing company every 12 to 18 months to see if there has been any change in the variant classification and/or change in personal/family history of cancer.
- Instruct LP to confirm with her gastroenterologist for recommended colonoscopy screening. The intervals should be based upon a personal and family history of colon polyps.
- Encourage LP to discuss menopausal symptoms with her health-care provider, as there are evidence-based interventions to manage post-HBSO side effects (Kaplan, 2021; Mahon & Carr, 2021; Singer, 2021).
- Reinforce cancer risk reduction and screening guidelines based on LP’s age and family history (not the VUS).
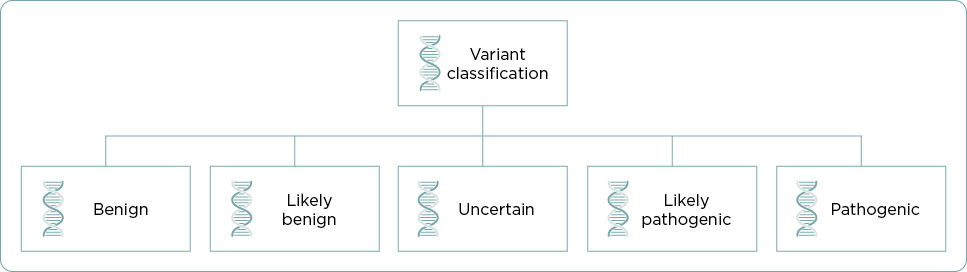
The term “variant” is applied to any alteration in the DNA sequence (National Cancer Institute [NCI], n.d.). A variant may be classified as a variant of uncertain significance (VUS) if there is conflicting evidence for one of the subcategories (benign, likely benign, likely pathogenic, or pathogenic classification), or if none of the criteria to up- or downgrade a variant are met (Richards et al., 2015). Due to multigene testing and expanded next-generation sequencing (NGS) testing, the rate of reported VUSs has increased exponentially, with some studies reporting a 15% to 32% frequency in patients who have genetic testing for hereditary cancer syndromes (Reuter et al., 2019). With hereditary breast and ovarian cancer genetic testing, a VUS is common and comprises approximately 40% of all reported variants (Federici & Soddu, 2020). The more genes that are on a testing panel, the more likely a VUS will be identified (NCCN, 2024). It is not surprising that LP had a VUS upon testing with an 81-gene panel.
The terms mutation, polymorphism, and variant have all been used to describe changes in DNA sequence often with the incorrect assumption that a mutation is pathogenic and a polymorphism is benign. To avoid confusion, the American College of Medical Genetics (ACMG) and the Association for Molecular Pathology (AMP) addressed the issue in the 2015 “Standards and Guidelines for the Interpretation of Sequence Variants” document (Richards et al., 2015). The ACMG/AMP recommendations replace the terms “polymorphism” and “mutation” with the more neutral term, “variant” including subclassifications such as benign, likely benign, uncertain, likely pathogenic, or pathogenic (Figure 2). The ACMG/AMP established criteria for each subcategory (Richards et al., 2015). A VUS may be referred to as either a variant of “uncertain significance” or a variant of “unknown significance” (NCI, n.d.).
VUS Medical Management
According to the ACMG/AMP Standards and Guidelines document in the section, “How Should Health-Care Providers Use These Guidelines and Recommendations?” (Richards et al., 2015, para 5), “a variant of uncertain significance should not be used in clinical decision making.” National Comprehensive Cancer Network (NCCN) Guidelines (2024) concur that a VUS alone should not alter medical management. It seems that LP’s health-care provider made a surgical management decision (hysterectomy and bilateral oophorectomy; HBSO) based upon an incomplete understanding of genetic testing and the PALB2 gene VUS.
The ACMG guidelines emphasize that, if possible, additional efforts should be made to classify a variant as benign or pathogenic. Because VUS reference data improve over time as more information is known, reclassification of a VUS to pathogenic or benign is possible (East et al., n.d.). Approximately 80% to 90% of VUSs are eventually reclassified as benign or likely benign, and 10% to 20% reclassified as pathogenic or likely pathogenic (NCCN, 2024). Variant of uncertain significance reference data are dynamic and should not be considered as “one and done” (Cyr, 2020). Genetics providers and patients should maintain contact at regular intervals to update contact information, determine if there have been changes in personal and family history of cancer, and ascertain if there has been a reclassification of the variant (Mueller et al., 2019). It is important to note that regardless of genetic testing results, personal and family history of cancer serve as the primary basis for screening and risk reduction interventions (NCCN, 2024).
The misinterpretation of a VUS can cause harm as illustrated in this case study where an HBSO was not medically indicated. Several publications over a period of years highlight the challenges and adverse events that can occur with the misinterpretation of cancer genetic testing results. These articles include examples of serious errors of omission and commission because of provider misinterpretation of VUS terminology (Bonadies et al., 2014; Farmer et al., 2019; Farmer et al., 2021). In one publication alone (Farmer et al., 2021), five of nine misinterpretation case reports were associated with a VUS, e.g., informing a patient that a VUS report was “negative” or recommending inappropriate screening or surgical management based upon a VUS. Concerning breast and ovarian cancers, Kurian and colleagues (2017) found that nearly half of surgeons did not understand the distinction between a VUS and a pathogenic variant and reported their management of patients with a BRCA1/2 VUS was the same as for a BRCA1/2 pathogenic variant. Liability concerning VUS interpretation and management is a challenge as reference databases are constantly changing, and there may be inconsistent variant classifications among testing laboratories (Marchant et al., 2020). Next steps in VUS management and interpretation include:
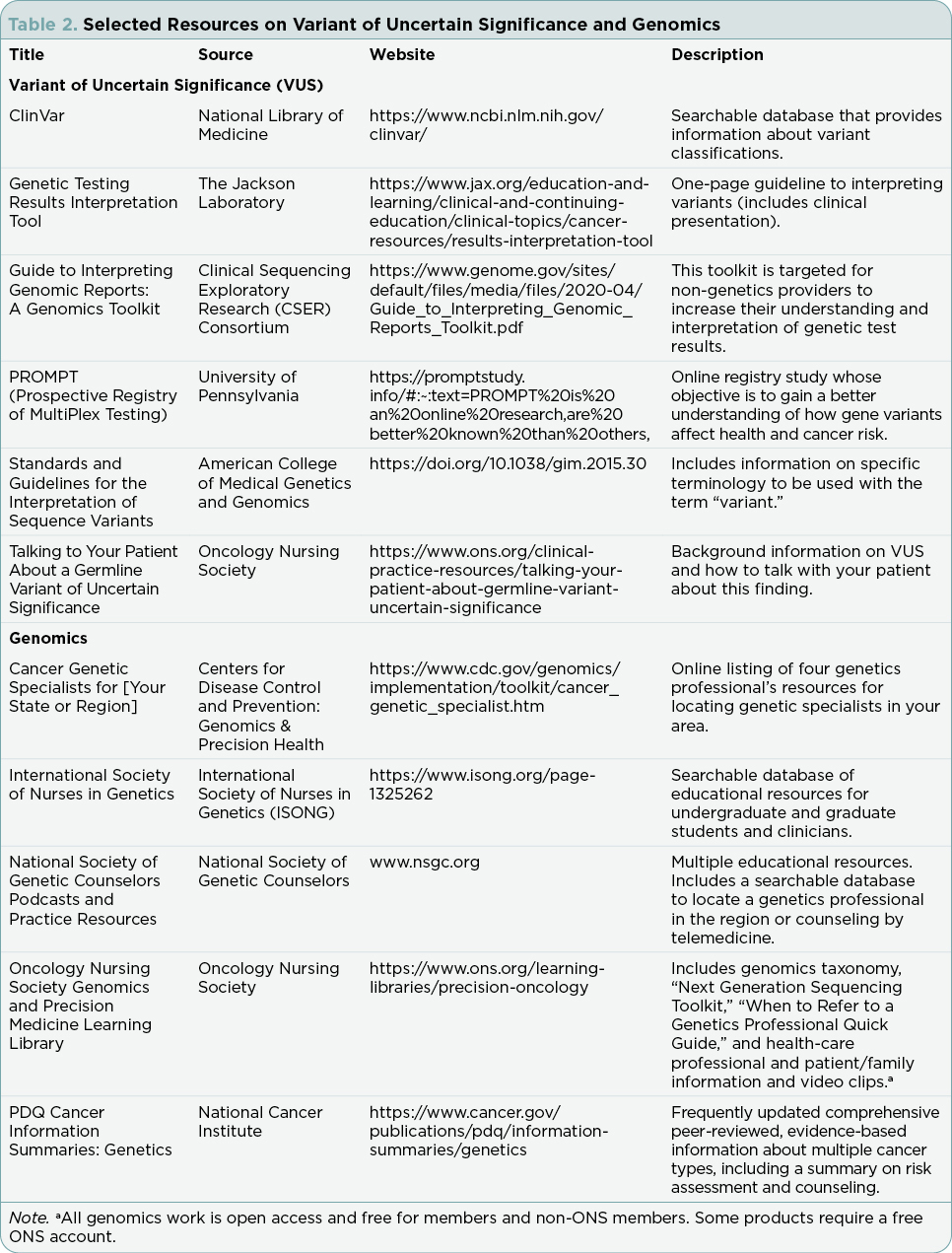
- Referral to genetic specialists listed in “Cancer Genetic Specialists for Your State or Region” (Table 2).
- Contact the laboratory to discuss the variant information and explore genetic variant resources such as ClinVar (Table 2).
- Identify appropriate research studies/registries, e.g., PROMPT registry (Table 2).
- Periodically assess the status (classification) of the variant (East et al., n.d.).
Genomic Literacy
The ACMG/AMP encourages professional societies to educate health-care professionals on how and when to use the “variant” terminology and the restrictions for using a VUS for medical management (Richards et al., 2015). In 2021, the Oncology Nursing Society (ONS) responded to the ACMG request for organizational involvement and issued a “Call to Action: Using the Appropriate Genomic Terminology for Safety and Quality,” challenging members and others to follow ACMG guidelines and use the preferred variant terminology in communications with patients, family members, and health-care professionals (ONS, n.d.). The Oncology Nursing Society also developed an online easily accessible genomics taxonomy to promote genomic literacy among oncology nurses (Friend et al., 2021). The NCI promotes the “variant” terminology on their physician data query (PDQ) website: “A concerted effort is being made within the genetics community to shift terminology used to describe genetic variation. The shift is to use the term ‘variant’ rather than the term ‘mutation’ to describe a difference that exists between the person or group being studied and the reference sequence, particularly for differences that exist in the germline.” (NCI, 2022, para 2). Resources are available to aid in the understanding and clinical application of VUS genetic test results (Table 2).
VUS Through the Eyes of the Patient
Making sense of an uncertain result can be unsettling for patients and family members, especially if the patient has not received pre-test counseling and informed consent. “We don’t know for sure” conversations are difficult. In this case study, LP did not have adequate pre-test counseling and did not understand there was a significant possibility the test results might indicate a VUS. LP was initially concerned about her risk for colon cancer. The PALB2 VUS was a complete surprise to her. LP was upset that she had unnecessary surgery and was experiencing HBSO adverse effects.
The literature suggests that a patient’s perception of a VUS is highly personal based on experiences, emotions, and individual informational needs (Reuter et al., 2019). Genetics providers should ask open-ended questions about the patient’s interpretation of the VUS and explore the patient’s personal factors and beliefs about genetics and cancer. For LP, her initial request for genetic testing was prompted by her personal and family history of colon polyps.
Analogies can be useful teaching tools in helping patients understand basic science concepts (Seiler & Huggins, 2018). For this case study, the G-APRN used a baking analogy to illustrate the meaning of a VUS, noting that a substitution in the recipe may or may not alter the final baking product (Mahon, 2022). Critical VUS messages should be communicated to patients:
- A VUS should not be used to guide surgical management or screening.
- VUS results do not necessarily explain a family history of cancer.
- VUS results may be reclassified in the future.
- The VUS does not rule out or explain other cancers present now or in the future (NCCN, 2024; Reuter et al., 2019).
Patients should be offered an opportunity to consult with a qualified genomics professional (Mahon & Yackzan, 2022) and participate in VUS studies such as the PROMPT (Prospective Registry of MultiPlex Testing) registry (Table 2), where patients can share VUS information and contribute to a better understanding of how variants affect health and cancer risks (University of Pennsylvania, 2022).
Summary
When a large multigene panel is ordered, there is a significant probability that one or more VUSs may be identified. Health-care providers should provide informed consent including anticipatory guidance for possible “uncertain” testing results, and when appropriate, consult with genetics professionals prior to testing to ensure that appropriate testing panels are ordered. A first step in addressing VUS literacy and understanding begins by using the correct “variant” terminology with the addition of variant qualifiers: benign, likely benign, uncertain, likely pathogenic, or pathogenic. For this case study, LP was referred to a G-APRN who addressed the VUS issues, provided client education, averted further unwarranted surgery, and mitigated potential liability for the current health-care team. Understanding and communicating information about the uncertainty and complexities of a VUS continues to be a challenge now and for the future. Genetic testing is complex; however, the promise of personalized health care is only possible when health-care professionals and patients and family members have an accurate interpretation of genetic testing results.
Disclosure
The authors have no conflicts of interest to disclose.
References
Bonadies, D. C., Brierley, K. L., Barnett, R. E., Baxter, M. D., Donenberg, T., Ducaine, W. L.,…Matloff, E. T. (2014). Adverse events in cancer GBT: The third case series. Cancer Journal, 20(4), 246–253. https://doi.org/10.1097/PPO.0000000000000057
Cyr, A. E. (2020). GBT is not “One and done”. Annals of Surgical Oncology, 27(7), 2114–2116. https://doi.org/10.1245/s10434-020-08493-8
East, K., Chung, W., Foreman, K., Gilmore, M., Gornick, M., Hindorff, L.,…Pion, S. (n.d.). Guide to interpreting genomic reports: A genomics toolkit. Clinical Sequencing Exploratory Research. https://www.genome.gov/sites/default/files/media/files/2020-04/Guide_to_Interpreting_Genomic_Reports_Toolkit.pdf
Farmer, M. B., Bonadies, D. C., Mahon, S. M., Baker, M. J., Ghate, S. M., Munro, C.,…Matloff, E. T. (2019). Adverse events in genetic testing: The fourth case series. Cancer Journal (Sudbury, Mass.), 25(4), 231–236. https://doi.org/10.1097/PPO.0000000000000391
Farmer, M. B., Bonadies, D. C., Pederson, H. J., Mraz, K. A., Whatley, J. W., Darnes, D. R.,…Matloff, E. T. (2021). Challenges and errors in genetic testing: The fifth case series. Cancer Journal, 27(6), 417–422. https://doi.org/10.1097/PPO.0000000000000553
Federici, G., & Soddu, S. (2020). Variants of uncertain significance in the era of high-throughput genome sequencing: A lesson from breast and ovary cancers. Journal of Experimental & Clinical Cancer Research, 39(1), 46. https://doi.org/10.1186/s13046-020-01554-6
Friend, P., Dickman, E., & Calzone, K. (2021). Using a genomics taxonomy: Facilitating patient care safety and quality in the era of precision oncology. Clinical Journal of Oncology Nursing, 25(2), 205–209. https://doi.org/10.1188/21.CJON.205-209
Kaplan, M. (2021). Sexual dysfunction: Common side effect. Clinical Journal of Oncology Nursing, 25(6), 16–20. https://doi.org/10.1188/21.CJON.S2.16-20
Kurian, A. W., Li, Y., Hamilton, A. S., Ward, K. C., Hawley, S. T., Morrow, M.,…Katz, S. J. (2017). Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. Journal of Clinical Oncology, 35(20), 2232–2239. https://doi.org/10.1200/JCO.2016.71.6480
Mahon, S. M. (2022). Understand genetic variants to confidently educate your patients. Oncology Nursing Society VOICE. https://voice.ons.org/news-and-views/understand-genomic-variants-to-confidently-educate-your-patients
Mahon, S. M., & Carr, E. (2021). Hot flashes: Common side effect. Clinical Journal of Oncology Nursing, 25(6), 28–28. https://doi.org/10.1188/21.CJON.S2.28
Mahon, S. M., & Yackzan, S. (2022). Oncology nurse practitioners in genetics: Examining scope of practice and competence. Clinical Journal of Oncology Nursing, 26(2), 141–145. https://doi.org/10.1188/22.CJON.141-145
Marchant, G., Barnes, M., Evans, J. P., LeRoy, B., & Wolf, S. M. (2020). From genetics to genomics: Facing the liability implications in clinical care. Journal of Law, Medicine & Ethics, 48(1), 11–43. https://doi.org/10.1177/1073110520916994
Mueller, A., Dalton, E., Enserro, D., Wang, C., & Flynn, M. (2019). Recontact practices of cancer genetic counselors and an exploration of professional, legal, and ethical duty. Journal of Genetic Counseling, 28(4), 836–846. https://doi.org/10.1002/jgc4.1126
National Cancer Institute. (n.d.). NCI Dictionary of Cancer Terms: Variant. https://www.cancer.gov/publications/dictionaries/cancer-terms/search/variant/?searchMode=Begins
National Cancer Institute. (2022). Genetics of Breast and Gynecologic Cancers (PDQ) – Health Professional Version, Introduction General Information. https://www.cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq#_1
National Center for Biotechnology Information. (2023). ClinVar NM_024675.4(PALB2):c.11C>T (p.Pro4Leu) [VCV000126593.22]. https://www.ncbi.nlm.nih.gov/clinvar/variation/126593/
National Comprehensive Cancer Network. (2023). NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal. V2.2023. https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1436
National Comprehensive Cancer Network. (2024). NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. V2.2024. https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503
National Library of Medicine. (n.d.). What is ClinVar? https://www.ncbi.nlm.nih.gov/clinvar/intro/
Oncology Nursing Society. (n.d.). Oncology Nursing Society Call to Action: Using the Appropriate Genomic Terminology for Safety and Quality. https://www.ons.org/sites/default/files/2021-11/ONS_Genomics_Terminology_Call_to_Action.pdf
Reuter, C., Chun, N., Pariani, M., & Hanson-Kahn, A. (2019). Understanding variants of uncertain significance in the era of multigene panels: Through the eyes of the patient. Journal of Genetic Counseling, 28(4), 878–886. https://doi.org/10.1002/jgc4.1130
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J.,…ACMG Laboratory Quality Assurance Committee. (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine, 17(5), 405–424. https://doi.org/10.1038/gim.2015.30
Seiler, K. P., & Huggins, J. (2018). From cheese curls to fatty acid structure: Using “commonplace” analogies to teach science to nonmajors. Advances in Physiology Education, 42(2), 393–395. https://doi.org/10.1152/advan.00180.2017
Singer, M. (2021). Osteoporosis: Common side effects. Clinical Journal of Oncology Nursing, 25(6), 13–15. https://doi.org/10.1188/21.CJON.S2.13-15
The Jackson Laboratory. (n.d.). Cancer Genetic Clinical Education. https://www.jax.org/education-and-learning/clinical-and-continuing-education/course-offerings/cancer-genetic-clinical-education
University of Pennsylvania. (2022). PROMPT. https://promptstudy.info/#:~:text=PROMPT%20is%20an%20online%20research,are%20better%20known%20than%20others,