Review Article
Role of Preemptive Cytomegalovirus Hyperimmunoglobulin in Cytomegalovirus Viremia Following Stem Cell Transplant: An Integrative Review
Jaci Whittaker, MSN, APRN, FNP-BC, Ashley Martinez, DNP, APRN, FNP-BC, and Joyce E. Dains, DrPH, JD, APRN, FNP-BC, FANP, FAANP, FAAN
From The University of Texas MD Anderson Cancer Center, Houston, Texas
Authors’ disclosures of conflicts of interest are found at the end of this article.
Correspondence to: Jaci Whittaker, MSN, APRN, FNP-BC, 5311 Larkin Street, Houston, TX 77007 E-mail: jschulz@mdanderson.org
J Adv Pract Oncol 2023;14(7):620–630 |
https://doi.org/10.6004/jadpro.2023.14.7.6 |
© 2023 BroadcastMed LLC

 ABSTRACT
ABSTRACT
Introduction: Cytomegalovirus (CMV) is a major cause of morbidity and mortality in stem cell transplant (SCT) patients. Cytomegalovirus hyperimmunoglobulin (CMV-HIG) therapy has been described in the solid organ transplant setting. However, no review has focused on preemptive use of intravenous CMV immunoglobulins in the SCT setting. This review aims to consolidate findings regarding the preemptive use of CMV-HIG for CMV viremia in SCT patients. Methods: PubMed and Scopus were searched using specific search criteria for publications from 2011 to 2021. Search terms were: cytomegalovirus, CMV, immunoglobulins, immunoglobulin, IVIG, CMVIG, hematopoietic stem cell transplantation, and stem cell. Included studies discussed stem cell transplantation, immunoglobulins, and cytomegalovirus. 366 articles were identified from the search. Five articles met the inclusion and exclusion criteria. Results: Preemptive CMV-HIG resulted in an overall response in 65% to 100% of patients with a clearance time of 14 to 21 days. Early use of CMV-HIG may shorten clearance time. No treatment-related mortality or serious adverse events were associated. Conclusion: CMV-HIG is an effective treatment option in SCT patients that is as safe as antivirals alone. Preemptive CMV-HIG with antivirals may provide the added advantage of reduced time to viremia clearance without adding renal injury. Larger, prospective studies are needed to evaluate CMV-HIG’s impact on time to viremia clearance and the effectiveness of preemptive CMV-HIG use with antivirals.
ARTICLE
Cytomegalovirus (CMV) is a major cause of morbidity and mortality in immunocompromised patients following stem cell transplant (SCT; Styczynski, 2017). Cytomegalovirus remains latent after primary infection and can reactivate in a host during a time of immune suppression. Due to immune suppression, SCT patients are among those at risk for CMV reactivation, causing CMV viremia. It has been estimated that the median rate of CMV recurrence in SCT patients is 37% (Styczynski, 2017). Identifying successful management of CMV viremia is important for minimizing mortality in this population.
Background
In recent years, there have been advancements in the use of antiviral medications for prophylactic and preemptive treatment of CMV viremia in SCT patients. Prophylaxis describes the administration of antiviral drugs to patients at risk of developing CMV disease following transplantation for a specified period of time in order to prevent infection (Gilioli et al., 2021; Goldstein et al., 2017; Meesing & Razonable, 2018). Preemptive therapy differs from prophylaxis as it describes a strategy in which antiviral drugs are given to patients with evidence of active CMV replication with viral loads above a threshold (Gilioli et al., 2021). High CMV viral load has been identified as a risk factor for death in SCT patients (Green et al., 2016). Cytomegalovirus DNA conversion, described as the time to negative CMV polymerase chain reaction (PCR) in blood or plasma, is typically confirmed with two consecutive tests and is used as a measure of successful CMV viremia treatment (Alsuliman et al., 2018; Camargo et al., 2018; Meesing & Razonable, 2018).
Intravenous CMV hyperimmunoglobulin (HIG) contains high concentrations of specific immunoglobulins targeted against CMV. Cytomegalovirus HIG is purified from donors recently vaccinated or recovering from CMV (Arumugham & Rayi, 2022. The goal of administration of CMV-HIG is to neutralize specific CMV antigens, thereby providing passive immunity to CMV to recipients (Arumugham & Rayi, 2022).
The utilization of CMV-HIG as a concomitant therapy has been described in the solid organ transplant setting (Meesing & Razonable, 2018; Schulz et al., 2016). Prior to 2011, there were two systematic reviews and meta-analyses focused on immunoglobulin prophylaxis in SCT (Raanani et al., 2008; Raanani et al., 2009). In 2018, a systematic review and meta-analysis was published focusing on the effectiveness of immunoglobulin prophylaxis in SCT patients (Ahn et al., 2018). There has not been a literature review focused on preemptive use of intravenous CMV-HIG. The purpose of this review is to consolidate findings in the recent literature regarding the preemptive use of CMV-HIG during treatment of CMV viremia in SCT patients and to identify situations when preemptive CMV-HIG could be beneficial.
Methods
A review of the literature was performed with the assistance of a research librarian utilizing PubMed and Scopus electronic databases. Search terms used were cytomegalovirus, CMV, immunoglobulins, immunoglobulin, IVIG, CMV-IG, hematopoietic stem cell transplantation, and stem cell. In order to capture recent publications, the search was limited to publications from 2011 to 2021.
Included studies were limited to those in English with human participants who had undergone an SCT and developed subsequent CMV viremia. All ages were included. Included studies discussed SCT, immunoglobulins, and cytomegalovirus. Studies were excluded if they were a duplicate, were focused on prophylaxis, did not discuss intravenous immunoglobulins, or were abstracts only. After a review of the abstract and/or the full text of the article, articles that focused on single end-organ involvement, cost, non-hyperimmune globulin products, or animal-derived immune globulin products were excluded. A total of five articles met the inclusion and exclusion criteria and are incorporated in this review (Figure 1).
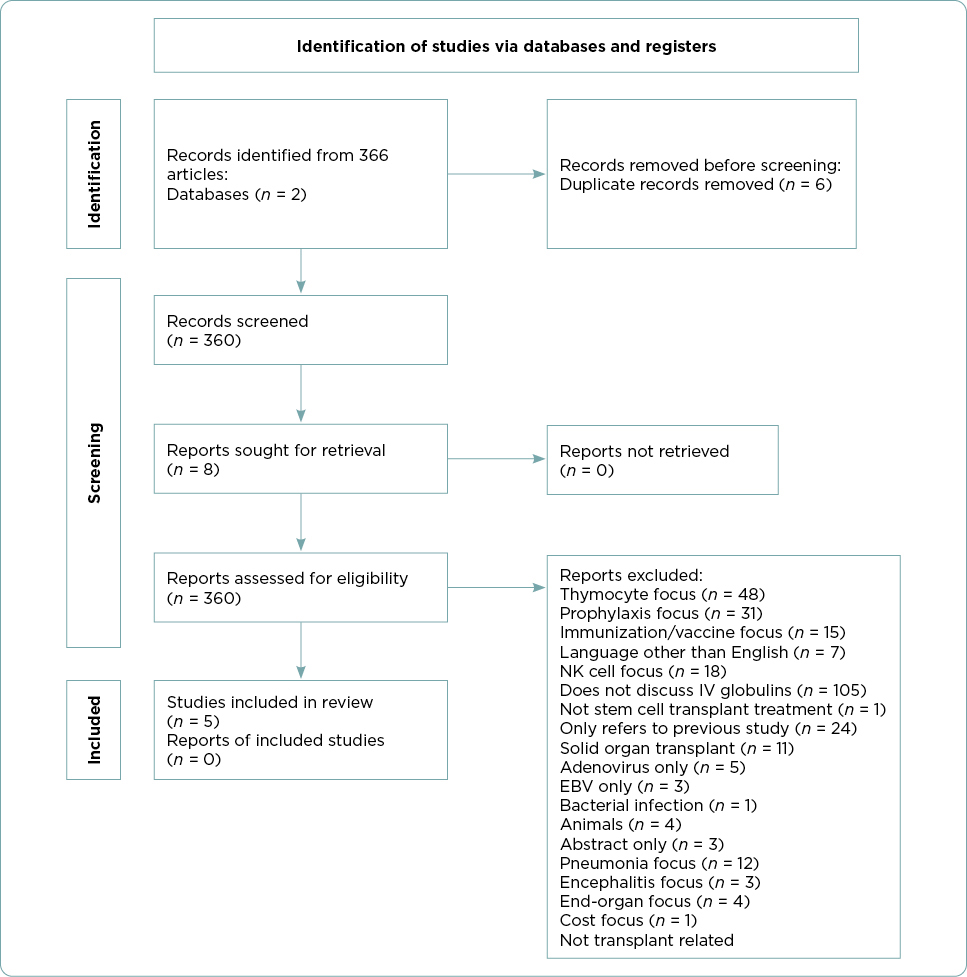
Results
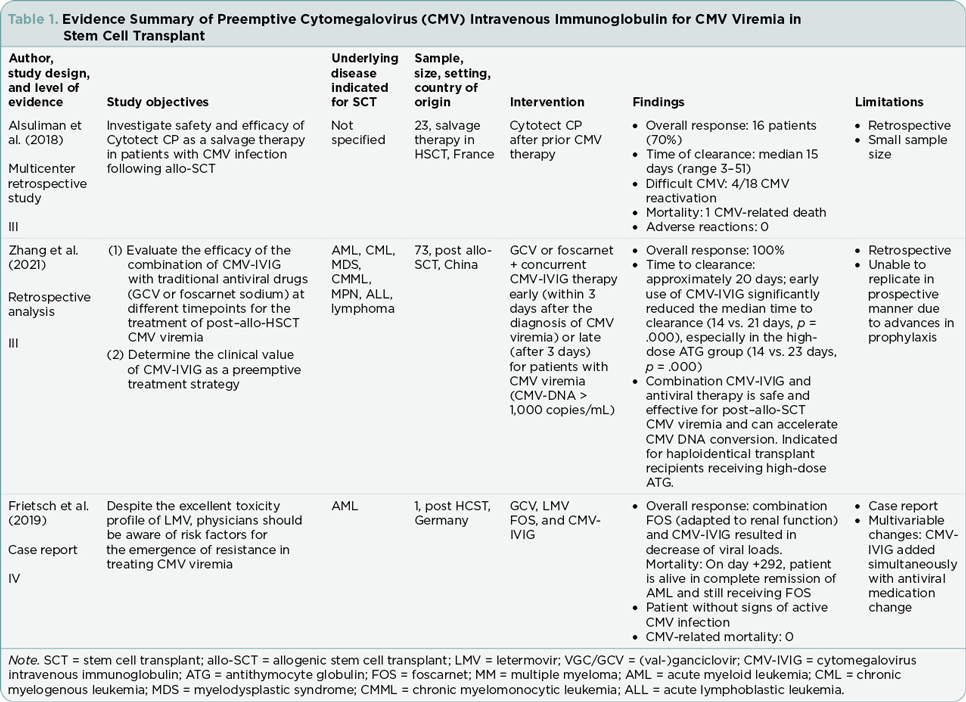
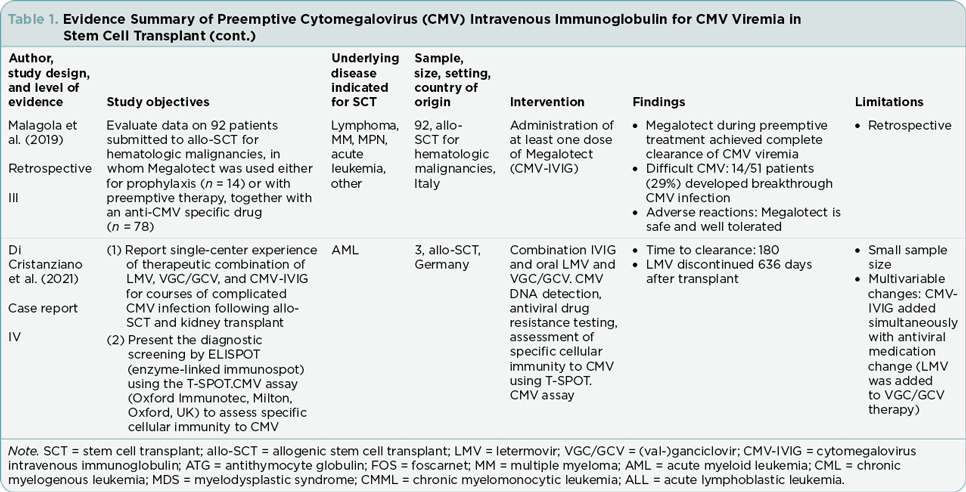
The five articles included three retrospective analyses and two case studies (Tables 1 and 2). Each of the five studies describe the effectiveness of combination antiviral therapy with CMV-HIG (Alsuliman et al., 2018; Di Cristanziano et al., 2021; Frietsch et al., 2019; Malagola et al., 2019; Zhang et al., 2021). Participant numbers across the studies ranged from one to 92. Retrospective analyses varied in duration from 9 months to 2 years. Although the studies included in this review varied in scope, focus, and specific interventions, the treatment outcomes evaluated at least one of the following areas that served as the organizing framework for this review: overall response rate, difficult CMV cases, time of CMV DNA clearance, transplant-related mortality, and adverse events.
Overall Response Rate
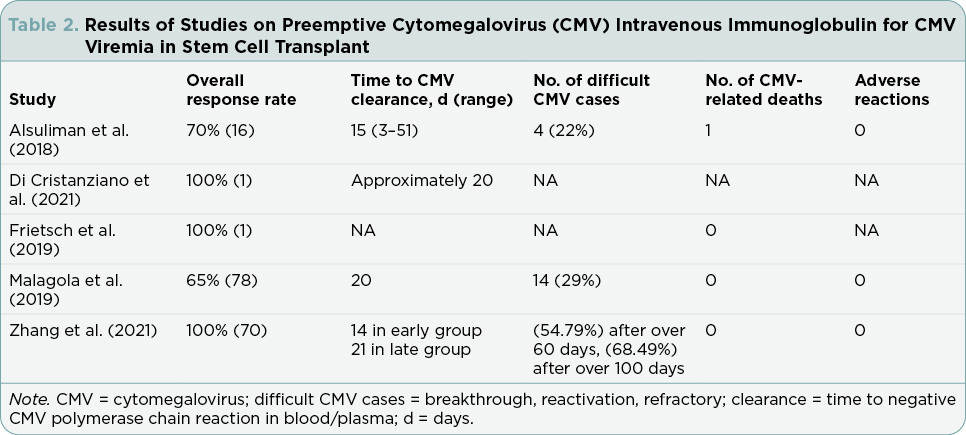
Overall response rate is defined as negative CMV PCR in the blood or plasma. All five articles discussed overall response rate with combined antiviral and CMV-HIG treatment (Alsuliman et al., 2018; Di Cristanziano et al., 2021; Frietsch et al., 2019; Malagola et al., 2019; Zhang et al., 2021). Only one (Alsuliman et al., 2018) reported on CMV-HIG monotherapy as well as combination therapy.
Zhang and colleagues (2021) reported on 70 post–allogeneic SCT patients experiencing CMV viremia between January and September 2020 in a hematology institute in China. In this retrospective analysis, the researchers evaluated the effectiveness of combination therapy of traditional antiviral drugs with CMV-HIG, comparing early intervention (CMV-HIG initiated within 3 days of diagnosis of CMV viremia) to late intervention (CMV-HIG initiated more than 3 days of diagnosis of CMV viremia). All patients (n = 70) were started on antiviral medication at diagnosis of CMV viremia. Treatment combinations included CMV-HIG given concurrently with the antivirals ganciclovir (GCV) or foscarnet sodium. The researchers reported that 100% of evaluable study participants (n = 70) exhibited overall response to treatment.
Malagola and colleagues (2019), in an Italian study in six bone marrow units, reported a more limited response to the incorporation of CMV-HIG in CMV viremia treatment. In the retrospective evaluation of 92 allogeneic SCT patients, Megalotect (CMV-HIG) was used as preemptive therapy for CMV viremia in combination with antiviral drugs (GCV, foscarnet, valganciclovir [VGC], or a two-drug combination) in 78 patients and as prophylactic therapy in 14 patients. An overall response was observed in 51/78 (65%) patients who received CMV-HIG as preemptive treatment.
Di Cristanziano and colleagues (2021) reported on three single-center transplant cases with prolonged CMV viremia in Germany. One of the three patients in their study underwent allogenic hematopoietic SCT for acute myeloid leukemia and developed VGC/GCV-resistant CMV viremia. Combination drug therapy comprised the antivirals letermovir and VGC/GCV, along with CMV-HIG. The researchers reported that this combination of antiviral drugs with CMV-HIG ultimately led to overall response.
In the study that included both combination therapy and monotherapy treatment with CMV-HIG alone, Alsuliman and colleagues (2018) specifically evaluated the safety and efficacy of the CMV-HIG product, Cytotect CP, in patients with CMV viremia following allogenic SCT. This retrospective study in France investigated 23 patients who had failed prior CMV therapy with antiviral medications. Cytotect CP was given as preemptive monotherapy in six patients and as prophylactic monotherapy in one patient. Preemptive combination therapy comprised Cytotect CP and antivirals (GCV, foscarnet sodium, combination GCV and foscarnet sodium, or other antiviral combinations) and was used in 16 patients. The researchers reported that 18 (78%) patients achieved response. A negative CMV PCR was achieved in 16 preemptively treated patients and the one prophylactically treated patient. One patient demonstrated a very good partial response. The study did not separately report the number of responders who received Cytotect CP as monotherapy or as combination therapy.
In a case study of a 60-year-old female with CMV infection following SCT for acute myeloid leukemia, Frietsch and colleagues (2019) reported a course that was complicated by delayed engraftment and mutations resistant to letermovir. Ultimately, combination therapy consisting of CMV-HIG and foscarnet led to a decrease in CMV DNA loads.
Time to CMV Clearance
Four of the articles reported the time of CMV DNA clearance or conversion (Alsuliman et al., 2018; Di Cristanziano et al., 2021; Malagola et al., 2019; Zhang et al., 2021). Time of CMV DNA clearance is described as the time to negative CMV PCR in blood or plasma and is used interchangeably with the term conversion.
In the 18 patients who responded to Cytotect CP as monotherapy or combination therapy, Alsuliman and colleagues (2018) reported a median conversion time of 15 days from initiation of Cytotect CP therapy. The range of days until conversion was from three to 51 days. Quantitative PCR (qPCR) was used to measure CMV clearance in blood.
Zhang and colleagues (2021) also identified a median clearance timeline of around 2 weeks in the retrospective study of 73 patients in China. Clearance of CMV was measured by real-time quantitative PCR (RT-qPCR) in blood. In this study, the median time to CMV clearance from treatment start time was 14 days in the early CMV-HIG therapy group (n = 27) and 21 days for the late CMV-HIG therapy group (n = 43). Early use of CMV-HIG significantly reduced the median time of CMV clearance (p = .000). The 2-week response rate was 63% (17/27) in the early CMV-HIG group and 16% (7/43) in the late CMV-HIG group.
While Alsuliman and colleagues (2018) and Zhang and colleagues (2021) reported conversion or clearance times of around 2 weeks, two other studies included in this literature review reported conversion times of 20 days. In the Italian study of 92 patients, Malagola and colleagues (2019) reported a 65% (51) clearance rate of CMV viremia at a median of 20 days. Clearance was measured by RT-qPCR in either plasma or whole blood. In the Di Cristanziano and colleagues (2021) single patient report, CMV DNA clearance was noted approximately 20 days following the initiation of CMV-HIG with letermovir and VGC/GCV therapy. Clearance was measured using CMV PCR in blood.
Difficult CMV Cases
Difficult CMV cases, including relapse, breakthrough, reactivation, or refractory CMV, were discussed in three of the five articles (Alsuliman et al., 2018; Malagola et al., 2019; Zhang et al., 2021). Relapse can be defined as a recurrence of a species that was present prior to therapy (Turck et al., 1968). Breakthrough CMV infection has been defined as the detection of CMV DNA in plasma while receiving prophylaxis (Klimczak-Tomaniak et al., 2020). Viral reactivation has been described as the recovery of a virus after a latency period (Cook & Trgovcich, 2011). Refractory CMV is defined as CMV viremia lasting longer than 2 weeks despite the administration of full-dose antiviral drug therapy (Chemaly et al., 2019).
The study by Alsuliman and colleagues (2018) was unique in describing CMV-HIG usage as preemptive monotherapy in six patients whose CMV infection had been refractory to at least two prior adequate lines of treatment. The researchers reported that of the 18 responders to CMV-HIG, four experienced CMV relapse. The timeframe for relapse was 9 to 49 days following the date of best response.
Although Malagola and colleagues (2019) in the Italian study did not report refractory CMV or drug-resistant CMV, the researchers reported that 74 (95%) of patients were CMV immunoglobulin G positive prior to allogenic SCT. Breakthrough CMV was reported in 29% (14/51) of responders following discontinuation of CMV-HIG therapy. Cytomegalovirus breakthrough occurred at a median of 30 days from CMV negativity.
In the study conducted in China, Zhang and colleagues (2021) reported that early use of CMV-HIG reduced the reactivation rate. Real-time qPCR monitored CMV DNA levels in serum, blood, and stool at least once weekly for 3 to 6 months following transplant. Although not statistically significant, early CMV-HIG therapy reduced the reactivation rate with 33 (45.21%) patients remaining negative for over 60 days, and 23 (31.51%) patients remaining negative for more than 100 days.
Transplant-Related Mortality Related to CMV
Transplant-related mortality was specifically reported in four of the five studies (Alsuliman et al., 2018; Frietsch et al., 2019; Malagola et al., 2019; Zhang et al., 2021). Transplant-related mortality describes death due to transplant-related causes other than disease relapse (Satwani et al., 2013). In the Alsuliman and colleagues (2018) study that included CMV-HIG monotherapy patients, five Cytotect CP treatment-responsive patients died within 100 days of treatment start. Of the five total deaths, one death was CMV related. Two additional patients who did not respond to Cytotect CP administration also died from other infection and graft-vs.-host disease (GVHD).
Malagola and colleagues (2019) reported 15% (13) of the 92 Italian study participants experienced transplant-related mortality. However, none of these deaths were attributable to CMV infection nor to CMV-HIG administration.
Zhang and colleagues (2021), who evaluated the efficacy of combination therapy of CMV-HIG with traditional antiviral drugs in 70 patients in China, also reported that no deaths occurred during the period of the study. Frietsch and colleagues (2019) in the single case reported that the patient was alive and without clinical signs of CMV infection at the time of publication.
Adverse Events
Adverse events were discussed by three of the five studies (Alsuliman et al. 2018; Malagola et al. 2019; Zhang et al., 2021). Each of the three studies reported that there were no adverse effects related to CMV-HIG administration.
Discussion
Marked positive overall response was described in each of the five studies. Passive immunity provided by CMV-HIG may explain this positive overall response (Arumugham & Rayi, 2022). The aim of hyperimmunoglobulins is to clear pathogens efficiently (Arumugham & Rayi, 2022). Studies comparing overall response in patients receiving only antiviral agents to patients receiving combination therapy of antivirals and CMV-HIG could better illuminate whether CMV-HIG improves overall response.
Details concerning overall response in the current studies offer considerations for future studies. Alsuliman and colleagues (2018) reported that 70% of the 23 patients in the study achieved an overall response. While the study included patients receiving monotherapy CMV-HIG as well as patients receiving combination therapy, the distinction of the two groups was not reported in the results of the study. Additionally, in the case study by Frietsch and colleagues (2019), after combination therapy with CMV-HIG, a decrease in CMV DNA loads was reported. However, the decrease in viral load is not quantified in the study.
Timing of the administration of CMV-HIG could contribute to overall response as well as the time to viremia clearance. Additionally, passive immunity may also help explain reduced time to viremia clearance. Hyperimmune globulins are rapid acting (Arumugham & Rayi, 2022). Zhang and colleagues (2021) found a significant reduction in median time of CMV clearance in patients who were in the early CMV-HIG therapy groups compared with those in late CMV-HIG therapy groups. Based on the findings by Zhang and colleagues (2021), preemptive use of CMV-HIG upon early detection of CMV may help decrease CMV viremia duration in SCT patients. Larger studies comparing time to clearance with use of preemptive CMV-HIG among various SCT populations are needed in the future.
Additionally, Frietsch and colleagues (2019) and Di Cristanziano and colleagues (2021) introduced CMV-HIG at the same time that antiviral medications were changed. Letermovir and CMV-HIG were concurrently added to VGC/GCV therapy (Di Cristanziano et al., 2021). Foscarnet and CMV-HIG were concurrently introduced following letermovir discontinuation (Frietsch et al., 2019). Due to simultaneous changes, the extent of improvement in CMV viral loads attributed to CMV-HIG cannot be determined. Studies containing a control group who receives antiviral treatment without CMV-HIG could be beneficial.
Cytomegalovirus HIG may have a role in difficult or reoccurring cases of CMV. Specifically, CMV-HIG may have a role in reducing CMV viremia relapse or achieving clearance of refractory CMV. Alsuliman and colleagues (2018), who included six patients with refractory CMV, reported that four of the 18 patients experienced relapse. However, the authors did not quantify how many relapsed cases were also refractory cases. Although not statistically significant, early CMV-HIG therapy reduced the reactivation rate in the study conducted by Zhang and colleagues (2021). Future studies that evaluate early CMV-HIG therapy could assess the duration of CMV viremia negativity without relapse and illuminate a role for CMV-HIG in difficult cases.
Safety, discussed in three of the articles, is a major consideration in therapy choice. Mortality and adverse events are important contributing factors in safe therapy choice. The results included in this literature review found no mortality related to CMV-HIG. The Cytotect CP study by Alsuliman and colleagues (2018) reported one death that was CMV viremia related, but not attributed to CMV-HIG. A study by Camargo and colleagues (2017) found that unresolved viremia by day 35 of antiviral treatment was associated with a significant increase in nonrelapse-related mortality. Based on findings by Zhang and colleagues (2021) that suggest preemptive CMV-HIG shortens time to viremia clearance, perhaps CMV-HIG could contribute to reducing mortality by reducing viremia duration. Additionally, no adverse events were reported by any of the studies. With no reported mortality and no adverse events, CMV-HIG can be considered a safe therapy choice.
Risks associated with therapy can be a component of safety as well. Kidney injury is a risk associated with some antivirals in SCT patients and can be a limiting factor in receiving specific antiviral treatments (Inose et al., 2022) The case study by Frietsch and colleagues (2019) describes the successful use of CMV-HIG in a patient who had experienced kidney injury secondary to foscarnet. Renally dose-adjusted foscarnet administration combined with CMV-HIG resulted in successful decrease of CMV viral loads (Frietsch et al., 2019). Additionally, the case study by Di Cristanziano and colleagues (2021) discusses the use of CMV-HIG in a patient who underwent allogeneic SCT with chemotherapy-induced kidney injury. He was not a candidate to receive foscarnet or cidofovir due to his kidney injury. Combination oral letermovir, VGC/GCV, and CMV-HIG achieved stable CMV viremia clearance (Di Cristanziano et al., 2021). Worsening kidney function in this case study is not mentioned by the authors, suggesting that perhaps CMV-HIG is safe to use in the setting of acute kidney injury. These two studies suggest that CMV-HIG may be safe for use in combination therapy in patients who have kidney injury. These outcomes contrast with expert opinion, which suggests that immunoglobulin preparations can cause kidney injury due to the high sucrose content in earlier preparations (El Helou & Razonable, 2019).
Limitations
Limitations of this review include small sample sizes, with two studies being case study designs. Additionally, each study in this literature review was retrospective in design and lacked control groups who did not receive CMV-HIG. Alsuliman and colleagues (2018) address the retrospective nature of their study and mentions that their study cannot be replicated in a prospective manner due to advances in prophylaxis and changes in treatment. Also, the antivirals used in the studies varied and included foscarnet, VGC, GCV, letermovir, and antiviral combinations. These antiviral variations could have impacted outcomes. Future studies with larger sample sizes, control groups, and prospective designs utilizing current antiviral recommendations would provide for more generalizable conclusions.
Implications for Practice
Duration of CMV Viremia
The findings in the study performed by Zhang and colleagues (2021) suggest that early, preemptive use of CMV-HIG shortens time to CMV viremia clearance. Early use of CMV-HIG significantly reduced CMV viremia clearance in patients who had received prophylactic antithymocyte globulin for GVHD. Clinicians may consider incorporation of preemptive CMV-HIG in patients when attempting to limit the duration of required antiviral therapy. Future prospective studies could evaluate time to CMV viremia clearance among SCT patients, accounting for patients on various GVHD prophylactic regimens.
Safety
As there were no reported mortalities nor adverse events in the studies in this literature review, clinicians can consider CMV-HIG as a safe therapy choice. Some expert opinions, as mentioned above, state that CMV-HIG is generally well tolerated but has been associated with adverse events (El Helou & Razonable, 2019). The CytoGam (CMV-HIG) package insert outlines general safety precautions for clinicians (Saol Therapeutics Research Limited, 2020). In clinical practice, the patient’s vital signs should be continuously monitored with observation for symptoms throughout the infusion. In the case of acute anaphylactic reaction, clinicians should ensure epinephrine and diphenhydramine are available (Saol Therapeutics Research Limited, 2020).
Conclusion
Based on the findings of this literature review, preemptive use of CMV-HIG with antivirals in SCT patients is an effective treatment option that is as safe as antivirals alone. Preemptive CMV-HIG use with antivirals may provide the added advantage of reduced time to viremia clearance without adding renal injury. Future, larger, prospective studies are needed to evaluate CMV-HIG’s impact on the time to viremia clearance and to evaluate the effectiveness of preemptive CMV-HIG use with antivirals.
Disclosure
The authors have no conflicts of interest to disclose.
References
Ahn, H., Tay, J., Shea, B., Hutton, B., Shorr, R., Knoll, G. A.,…Cowan, J. (2018). Effectiveness of immunoglobulin prophylaxis in reducing clinical complications of hematopoietic stem cell transplantation: A systematic review and meta-analysis. Transfusion, 58(10), 2437–2452. https://doi.org/10.1111/trf.14656
Alsuliman, T., Kitel, C., Dulery, R., Guillaume, T., Larosa, F., Cornillon, J.,…Yakoub-Agha, I. (2018). Cytotect®CP as salvage therapy in patients with CMV infection following allogeneic hematopoietic cell transplantation: A multicenter retrospective study. Bone Marrow Transplantation, 53(10), 1328–1335. https://doi.org/10.1038/s41409-018-0166-9
Arumugham, V. B., & Rayi, A. (2022). Intravenous immunoglobulin (IVIG). In StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK554446/#
Camargo, J. F., Kimble, E., Rosa, R., Shimose, L. A., Bueno, M. X., Jeyakumar, N.,…Komanduri, K. V. (2018). Impact of cytomegalovirus viral load on probability of spontaneous clearance and response to preemptive therapy in allogeneic stem cell transplantation recipients. Biology of Blood and Marrow Transplantation, ٢٤(4), 806–814. https://doi.org/10.1016/j.bbmt.2017.11.038
Chemaly, R. F., Chou, S., Einsele, H., Griffiths, P., Avery, R., Razonable, R. R.,…Resistant Definitions Working Group of the Cytomegalovirus Drug Development Forum. (2019). Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clinical Infectious Diseases, 68(8), 1420–1426. https://doi.org/10.1093/cid/ciy696
Cook, C. H., & Trgovcich, J. (2011). Cytomegalovirus reactivation in critically ill immunocompetent hosts: A decade of progress and remaining challenges. Antiviral Research, 90(3), 151–159. https://doi.org/10.1016/j.antiviral.2011.03.179
Di Cristanziano, V., Affeldt, P., Trappe, M., Wirtz, M., Heger, E., Knops, E.,…Grundmann, F. (2021). Combined therapy with intravenous immunoglobulins, letermovir and (val-)ganciclovir in complicated courses of CMV-infection in transplant recipients. Microorganisms, 9(8), 1666. https://doi.org/10.3390/microorganisms9081666
El Helou, G., & Razonable, R. R. (2019). Safety considerations with current and emerging antiviral therapies for cytomegalovirus infection in transplantation. Expert Opinion on Drug Safety, 18(11), 1017–1030. https://doi.org/10.1080/14740338.2019.1662787
Frietsch, J. J., Michel, D., Stamminger, T., Hunstig, F., Birndt, S., Schnetzke, U.,…Hilgendorf, I. (2019). In vivo emergence of UL56 C325Y cytomegalovirus resistance to letermovir in a patient with acute myeloid leukemia after hematopoietic cell transplantation. Mediterranean Journal of Hematology and Infectious Diseases, 11(1), e2019001. https://doi.org/10.4084/MJHID.2019.001
Gilioli, A., Messerotti, A., Bresciani, P., Cuoghi, A., Pioli, V., Colasante, C.,…Narni, F. (2021). Cytomegalovirus reactivation after hematopoietic stem cell transplant with CMV-IG prophylaxis: A monocentric retrospective analysis. Journal of Medical Virology, 93(11), 6292–6300. https://doi.org/10.1002/jmv.26861
Goldstein, G., Rutenberg, T. F., Mendelovich, S. L., Hutt, D., Oikawa, M. T., Toren, A., & Bielorai, B. (2017). The role of immunoglobulin prophylaxis for prevention of cytomegalovirus infection in pediatric hematopoietic stem cell transplantation recipients. Pediatric Blood & Cancer, 64(7), e26420. https://doi.org/10.1002/pbc.26420
Green, M. L., Leisenring, W., Xie, H., Mast, T. C., Cui, Y., Sandmaier, B. M.,…Boeckh, M. (2016). Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of preemptive therapy: A retrospective cohort study. The Lancet Haematology, 3(3), e119–e127. https://doi.org/10.1016/S2352-3026(15)00289-6
Inose, R., Takahashi, K., Takahashi, M., Sugimoto, T., Nanno, S., Hino, M., & Nagayama, K. (2022). Long-term use of Foscarnet is associated with an increased incidence of acute kidney injury in hematopoietic stem cell transplant patients: A retrospective observational study. Transplant Infectious Disease, 24(2), e13804. https://doi.org/10.1111/tid.13804
Klimczak-Tomaniak, D., Roest, S., Brugts, J. J., Caliskan, K., Kardys, I., Zijlstra, F.,…Manintveld, O. C. (2020). The association between cytomegalovirus infection and cardiac allograft vasculopathy in the era of antiviral valganciclovir prophylaxis. Transplantation, 104(7), 1508–1518. https://doi.org/10.1097/TP.0000000000003015
Malagola, M., Greco, R., Santarone, S., Natale, A., Iori, A. P., Quatrocchi, L.,…Peccatori, J. (2019). CMV Management with specific immunoglobulins: A multicentric retrospective analysis on 92 allotransplanted patients. Mediterranean Journal of Hematology and Infectious Diseases, 11(1), e2019048. https://doi.org/10.4084/MJHID.2019.048
Meesing, A., & Razonable, R. R. (2018). New developments in the management of cytomegalovirus infection after transplantation. Drugs, 78(11), 1085–1103. https://doi.org/10.1007/s40265-018-0943-1
Raanani, P., Gafter-Gvili, A., Paul, M., Ben-Bassat, I., Leibovici, L., & Shpilberg, O. (2008). Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. The Cochrane Database of Systematic Reviews, (4), CD006501. https://doi.org/10.1002/14651858.CD006501.pub2
Raanani, P., Gafter-Gvili, A., Paul, M., Ben-Bassat, I., Leibovici, L., & Shpilberg, O. (2009). Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: Systematic review and meta-analysis. Journal of Clinical Oncology, 27(5), 770–781. https://doi.org/10.1200/JCO.2008.16.8450
Saol Therapeutics Research Limited. (2020). CytoGam cytomegalovirus immune globulin intravenous (human). US prescribing information. https://www.fda.gov/media/77671/download
Satwani, P., Jin, Z., Duffy, D., Morris, E., Bhatia, M., Garvin, J. H.,…Cairo, M. S. (2013). Transplantation-related mortality, graft failure, and survival after reduced-toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biology of Blood and Marrow Transplantation, 19(4), 552–561. https://doi.org/10.1016/j.bbmt.2012.12.005
Schulz, U., Solidoro, P., Müller, V., Szabo, A., Gottlieb, J., Wilkens, H., & Enseleit, F. (2016). CMV immunoglobulins for the treatment of CMV infections in thoracic transplant recipients. Transplantation, 100(Suppl 3), S5–S10. https://doi.org/10.1097/TP.0000000000001097
Styczynski, J. (2017). Who is the patient at risk of CMV recurrence: A review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infectious Diseases and Therapy, 7(1), 1–16. https://doi.org/10.1007/s40121-017-0180-z
Turck, M., Ronald, A. R., & Petersdorf, R. G. (1968). Relapse and reinfection in chronic bacteriuria. II. The correlation between site of infection and pattern of recurrence in chronic bacteriuria. The New England Journal of Medicine, 278(8), 422–427. https://doi.org/10.1056/NEJM196802222780804
Zhang, P., Yang, D., Tian, J., Feng, S., Jiang, E., & Han, M. (2021). A clinical study of lyophilized intravenous human immunoglobulin containing high-titer cytomegalovirus-neutralizing antibody for the treatment of cytomegalovirus viremia after allogeneic hematopoietic stem cell transplantation. Annals of Palliative Medicine, 10(5), 5533–5540. https://doi.org/10.21037/apm-21-1069