Review Article
Impact of Fasting on Patients With Cancer: An Integrative Review
Samantha Thompson, MPH, MCHS, PA-C, Lydia T. Madsen, PhD, RN, AOCNS®, FNAP, and Angela Bazzell, DNP, APRN, FNP-BC, AOCNP®
From Harold C. Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center, Dallas, Texas
Authors’ disclosures of conflicts of interest are found at the end of this article.
Correspondence to: Samantha Thompson, MPH, MCHS, PA-C, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390. E-mail: samantha.thompson@utsouthwestern.edu
J Adv Pract Oncol 2023;14(7):608–619 |
https://doi.org/10.6004/jadpro.2023.14.7.5 |
© 2023 BroadcastMed LLC

 ABSTRACT
ABSTRACT
Background: Patients with cancer often pursue nutrition as an avenue to positively impact their care management and disease outcomes. Nutritional interventions are increasing in popularity, especially intermittent fasting as an adjunct to chemotherapy. However, limited research is available on the impact of intermittent fasting on patients with cancer. Methods: A comprehensive literature search was conducted using Ovid MEDLINE, Ovid EMBASE, and CINAHL databases. Results: 514 articles were identified from the three databases. Seven studies remained after applying inclusion and exclusion criteria. The seven studies included in this review examined fasting compliance, malnutrition, therapy side effects, endocrine parameters, quality of life measures, and cancer outcomes. Data suggest overall good compliance, no malnutrition, minimal side effects, a trend toward improved endocrine parameters, unchanged quality of life (QOL), and mixed results for cancer outcomes. Conclusion: Intermittent fasting as an adjunct to chemotherapy in normal-weight patients with cancer has potential as a safe, tolerable, and feasible nutritional intervention that could positively impact treatment outcomes and QOL. Large-scale randomized controlled trials are needed to validate these findings and determine what future role intermittent fasting may play in cancer management.
ARTICLE
Beyond the latest fad diet capturing the public’s attention, the practice of intermittent fasting has been advanced as a dietary intervention with the potential to positively impact cancer treatment by decreasing chemotherapy-related side effects and limiting tumor growth (Nencioni et al., 2018; Tinsley & La Bounty, 2015). Nutritional interventions involving fasting are an emerging area of interest among patients with cancer. As many as 63% of patients with cancer in a study of 109 participants, and 98% of cancer survivors among a study of 1,073 participants, consider nutrition to be an important part of their cancer management (Lee et al., 2018; Sullivan et al., 2021). Additionally, 48% of patients with cancer identified in a literature review that included 19,416 participants reported actively seeking health benefits from special diets as a way to improve disease outcomes (Zick et al., 2018).
Within the larger US adult population, approximately 52% of Americans aged 18 to 34, and 33% aged 35 to 80, followed a special diet in 2021, with intermittent fasting reported as one of the most common eating patterns used (International Food Information Council, 2021). Given this interest, a limited number of studies have incorporated intermittent fasting as a nutritional intervention in conjunction with chemotherapy (Bauersfeld et al., 2018; de Groot et al., 2020; Dorff et al., 2016; Lugtenberg et al., 2021; Riedinger et al., 2020; Tang et al., 2021; Zorn et al., 2018).
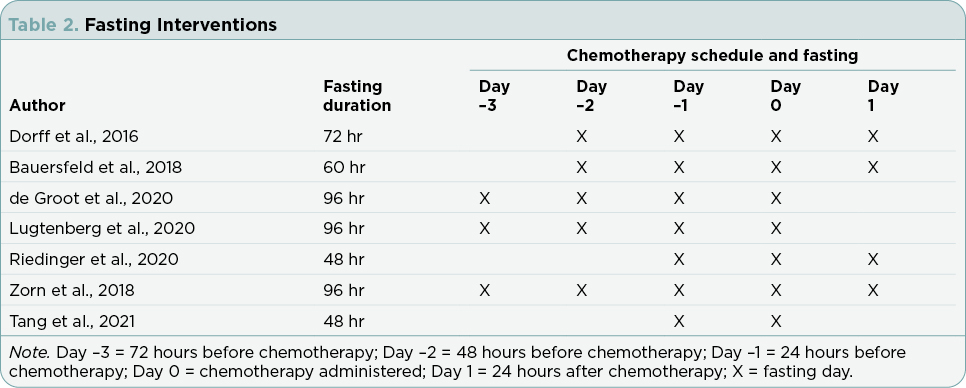
Intermittent fasting encompasses a wide spectrum of dietary patterns that involve periods of calorie restriction or calorie reduction over a specified time interval (Tinsley & La Bounty, 2015). The limitations placed upon calorie consumption varies greatly among dietary patterns (Tinsley & La Bounty, 2015). Short-term calorie restriction (SCR) consists of a strict water-only fasting period with no calories permitted from food, liquids, or intravenous (IV) fluids (Tang et al., 2021). Short-term fasting (STF) and short-term starvation (STS) restrict all calories from food along with no or very limited calories from liquids during designated fasting hours (Bauersfeld et al., 2018; Dorff et al., 2016; Riedinger et al., 2020). The fasting mimicking diet (FMD) and modified short-term fasting (mSTF) utilize calorie reduction to achieve a 25% to 50% deficit in total daily calories consumed from food and liquids throughout the fasting period, minimizing the risk of malnutrition from decreased calorie intake (de Groot et al., 2020; Klement & Champ, 2014; Lugtenberg et al., 2021; Omodei & Fontana, 2011; Zorn et al., 2018).
The duration of the fasting period also differs among dietary patterns, lasting 12 hours or more at a time (Longo & Mattson, 2014; Lugtenberg et al., 2021; Tinsley & La Bounty, 2015). When fasting is used as an adjunct to chemotherapy, a minimum fasting period of at least 48 hours is currently recommended for nutritional interventions in order to achieve a measurable metabolic response at the cellular level (Ketelslegers et al., 1996; Mansell & Macdonald, 1990; Raffaghello et al., 2008; Romijn et al., 1990; Safdie et al., 2012).
Given the variation among the multiple versions of intermittent fasting and the increasing interest of patients with cancer for nutritional interventions, oncology advanced practitioners need evidence-based information on the impact of fasting on patients with cancer to guide clinical education and decision-making. The purpose of this review is to examine the most recent evidence on the impact of intermittent fasting regimens on patients with cancer while undergoing systemic therapy.
Methods
A comprehensive search of the available literature was conducted on December 10, 2021, using three electronic databases: Ovid MEDLINE, Ovid Excerpta Medica Database (EMBASE), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). The search strategy was developed with the assistance of a research librarian and is available in a supplemental file. The search utilized a combination of Medical Subject Headings (MeSH) terms and keywords related to cancer, fasting, and chemotherapy while following the Population, Intervention, Comparison, Outcome (PICO) framework (Brown, 2020). Inclusion criteria consisted of patients with a cancer diagnosis undergoing fasting interventions during systemic therapy. Cancer was defined as any malignant neoplasm. Fasting interventions included periods of calorie restriction or reduction for at least 48 hours during chemotherapy. Systemic therapy was specifically defined as either chemotherapy or concurrent chemotherapy with immunotherapy.
Articles published prior to 2016 or not peer reviewed were excluded. Study designs utilizing variable fasting periods for religious reasons or fasting periods less than 48 hours were omitted. Only randomized control trials and prospective cohort studies were included to select for high-quality study designs with strong levels of evidence (Burns et al., 2011). Publications were limited to English language, human subjects, and adult populations.
A systematic approach was used to identify and screen research studies following the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1; Page et al., 2021). A total of 514 articles were identified in the initial literature search. After exclusion of duplicates using EndNote, 477 articles remained. Articles were screened for relevance first by title and then by abstract. Seven articles remained that met all inclusion and exclusion criteria. After completing a full-text review, the seven research articles were included in this review. The reference list of each study was also examined for additional articles of relevance, but no additional articles were identified.
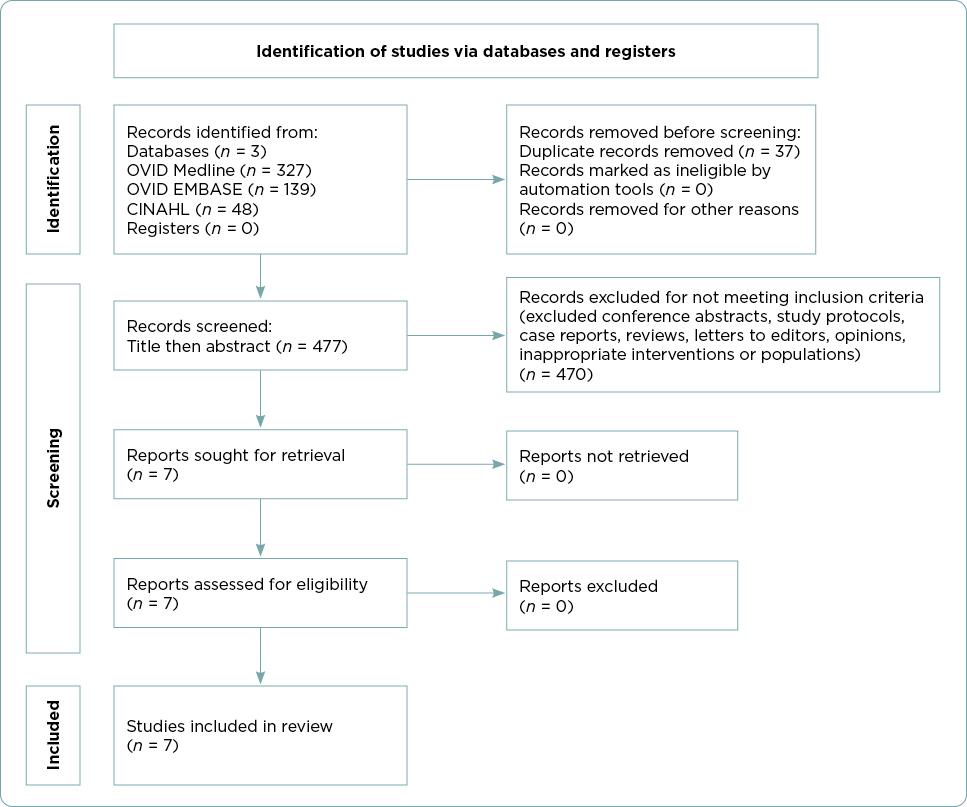
Results
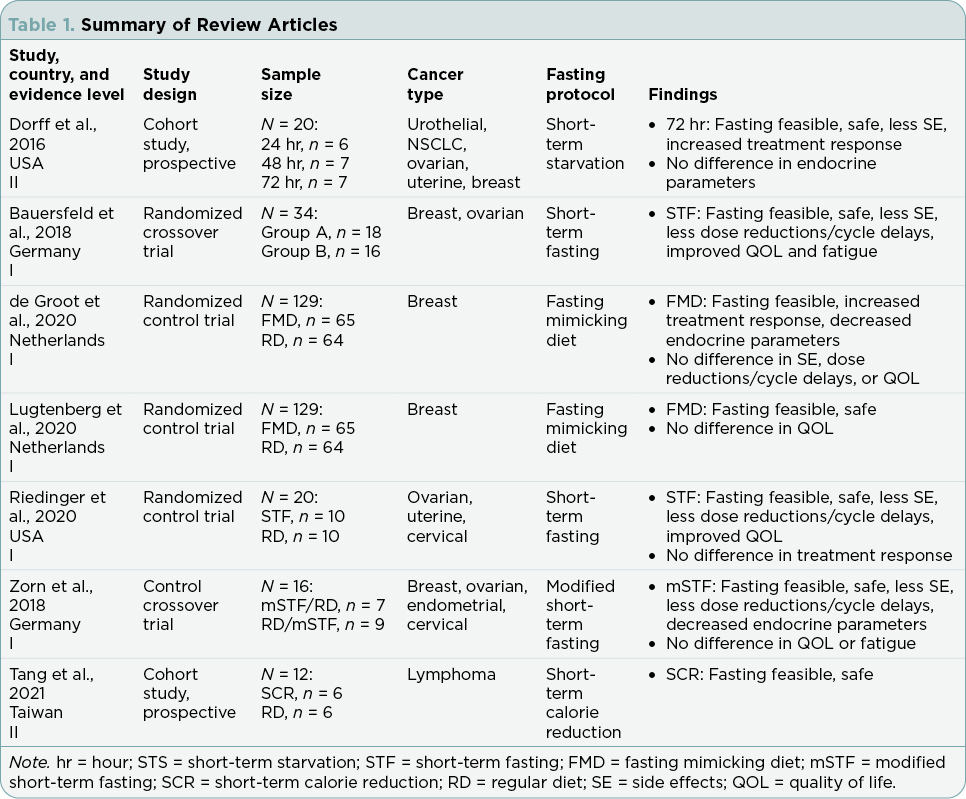
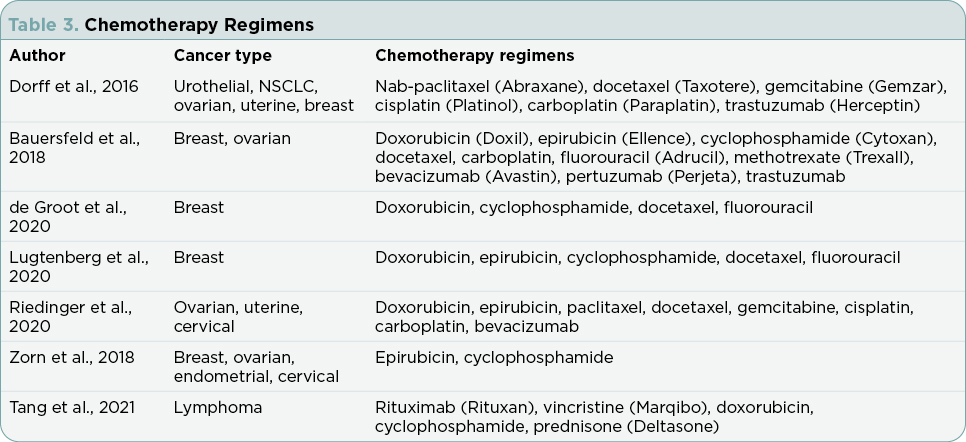
Research articles meeting inclusion and exclusion criteria included in this review addressed compliance and results for the following outcomes: fasting interventions, malnutrition, therapy side effects, endocrine parameters, quality-of-life (QOL) measures, and cancer outcomes. Table 1 summarizes the findings from the seven studies. Table 2 defines the nutritional interventions identified by the seven studies. Table 3 outlines chemotherapy regimens reported among the seven studies.
Compliance With Fasting Intervention
There are potential benefits and drawbacks when integrating a fasting protocol with cancer treatment. Some patients with cancer use fasting as a coping mechanism (Mas et al., 2019). Alternatively, fasting may offer patients a sense of control over one aspect of their cancer treatment, leading to less anxiety and possibly increasing the potential for compliance (Mas et al., 2019; Tang et al., 2021). However, adherence to a fasting regimen during chemotherapy may also present a burden to some patients with cancer. Dietary restrictions may be difficult to follow or sustain and are time-consuming, increasing the potential for poor compliance (Nencioni et al., 2018; Tang et al., 2021).
All seven studies included fasting compliance in some capacity with an overall theme of compliance reported among the majority of participants. Tang and colleagues’ (2021) was the only study to report 100% participant compliance with fasting during every cycle of chemotherapy. The authors attributed the high compliance rate to participants having access to research staff through a social networking application and the brief 48-hour duration of the fasting protocol (Tang et al., 2021). Riedinger and colleagues (2020) noted that participants were compliant with fasting for 95% of their chemotherapy cycles. An overall fasting compliance rate of 65% was observed by Dorff and colleagues (2016), with forgetfulness and social constraints given as the most common reasons for fasting noncompliance. Bauersfeld and colleagues (2018) documented fasting compliance during chemotherapy as adequate but did not provide a compliance percentage for reference. According to Zorn and colleagues (2018), participants in study arms A and B maintained a mean daily caloric intake consistent with the study fasting protocol, but compliance rates were not recorded.
Two separate studies analyzed data from the same clinical trial, describing identical overall fasting compliance rates. Both de Groot and colleagues (2020) and Lugtenberg and colleagues (2021) reported a fasting compliance rate of 21.5% among participants during all chemotherapy
cycles. Trial participants were 81.5% compliant during chemotherapy cycle 1, over 50% during chemotherapy cycle 2, and 33.8% during at least 4 cycles of chemotherapy (Lugtenberg et al., 2021). Reasons given for noncompliance included taste (51.0%), nausea (15.4%), hunger (9.8%), discontinuation of chemotherapy (3.9%), and other unspecified issues (35.3%; Lugtenberg et al., 2021). Of note, 7.8% of participants in the control group were noncompliant with a normal diet during one or more cycles of chemotherapy (de Groot et al., 2020; Lugtenberg et al., 2021). These individuals were deemed noncompliant for participating in fasting during chemotherapy (Lugtenberg et al., 2021).
Malnutrition
Up to 85% of patients with cancer experience malnutrition and/or weight loss (Bozzetti et al., 2012; Hébuterne et al., 2014), which increases the risk for treatment side effects, reduces quality of life, and accounts for up to 20% of cancer deaths (Muscaritoli et al., 2021). The increased risk for poor outcomes associated with malnutrition, weight loss, and cachexia poses an obvious safety concern for patients with cancer who participate in calorie-restricted fasting (Arends et al., 2017a; Arends et al., 2017b; Dirks & Leeuwenburgh, 2006; Longo & Mattson, 2014; Nencioni et al., 2018). Long-term fasting, although documented to reflect positive metabolic changes at the cellular level, may be associated with malnutrition and significant weight loss (Brandhorst et al., 2015; Lee & Longo, 2011; Lugtenberg et al., 2021; Tang et al., 2021). However, short-term fasting involving water-only or limited daily calorie consumption for less than a week has the potential to achieve positive metabolic changes while avoiding malnutrition and significant weight loss (Brandhorst et al., 2015; D’Aronzo et al., 2015; Lo Re et al., 2018; Nencioni et al., 2018; Panebianco et al., 2017). Furthermore, weight loss during short-term fasting is mostly attributed to fluid loss, with an average of 0.3 to 0.9 kg/day and is generally regained after normal diet is resumed, limiting the risk for significant weight loss (Kerndt et al., 1982; Naveed et al., 2014; Riedinger et al., 2020).
Six of the studies addressed the safety of fasting in cancer patients by monitoring indicators of malnutrition. No significant changes were identified to suggest increased harm. Three studies noted stable weight with minimal fluctuation after the completion of fasting in conjunction with chemotherapy (Bauersfeld et al., 2018; Riedinger et al., 2020; Tang et al., 2021). Dorff and colleagues (2016) did not observe malnutrition among their participants. Zorn and colleagues (2018) documented a weight loss of < 5% during fasting, which was significant (p = .002) compared with normal diet weight. A significant decrease in fat mass (p = .008) was also observed among fasting participants (Zorn et al., 2018). However, lean body mass was unchanged and body composition remained stable in fasting participants (Zorn et al., 2018). Lugtenberg and colleagues (2021) reported a modest decline in body mass index (BMI) among fasting participants; however, the participants maintained a normal stable weight 6 months after study completion. While de Groot and colleagues (2020) utilized the same data as Lugtenberg and colleagues (2021), weight loss was not addressed as a variable in their findings.
Therapy Side Effects
Chemotherapy is associated with acute and long-term side effects that may negatively impact treatment protocols (Cleeland et al., 2012; Dorff et al., 2016; Nencioni et al., 2018). Side effects are often severe enough to warrant dose reductions, cycle delays, early discontinuation of regimens, and possibly hospitalization, ultimately limiting the amount of chemotherapy administered and increasing the risk for poor outcomes (Cleeland et al., 2012; Dorff et al., 2016; Nencioni et al., 2018; Zorn et al., 2018). Patients with cancer are therefore motivated by the potential for fasting to limit the negative side effects of chemotherapy (Mas et al., 2019).
Chemotherapy-Induced Toxicities. Five studies examined the severity of chemotherapy-induced toxicities among fasting participants, with the majority identifying none to low frequency of severe toxicities. Toxicities were defined using the Common Terminology Criteria for Adverse Events (CTCAE; National Cancer Institute, 2022). Two studies failed to observe any CTCAE grade 3 and 4 toxicities among fasting participants (Bauersfeld et al., 2018; Riedinger et al., 2020). Zorn and colleagues (2018) documented CTCAE grade 3 nausea in a single participant during fasting and no CTCAE grade 4 toxicities during fasting. Furthermore, the frequency and severity score of the total self-reported toxicities among participants was significantly lower during fasting compared with the normal diet (p = .023; Zorn et al., 2018). Dorff and colleagues (2016) found fewer CTCAE grade 3 and 4 toxicities among participants fasting for 72 hours compared with participants fasting for 24 or 48 hours. There were fewer cases of CTCAE grade 3 and 4 neutropenia among participants fasting for 48 hours and 72 hours compared with participants fasting for 24 hours; however, this finding was not statistically significant (p = .17; Dorff et al., 2016). de Groot and colleagues (2020) reported no significant difference in CTCAE grade 3 and 4 neutropenic fever and neutropenia between fasting and regular diet. However, it should be noted that the lack of significance may have been masked given that regular diet participants received dexamethasone to prevent chemotherapy-induced toxicities, while fasting participants were not offered dexamethasone (de Groot et al., 2020).
Chemotherapy Tolerability. Four studies explored the impact of chemotherapy-induced toxicities on chemotherapy tolerability while fasting. The majority of studies found fewer chemotherapy dose reductions or cycle delays among the fasting participants when compared with the control group. Two studies reported high tolerance for chemotherapy among fasting participants (Bauersfeld et al., 2018; Riedinger et al., 2020). Zorn and colleagues (2018) documented a statistically significant decrease in the number of chemotherapy delays during fasting compared with normal diet (p = .034). On the contrary, de Groot and colleagues (2020) failed to identify a significant difference in the number of chemotherapy cycles stopped due to chemotherapy-induced toxicities between fasting and regular diet.
Endocrine Parameters
Fasting results in a significant decrease in several key hormones circulating in the cell plasma (Di Biase & Longo, 2016; Lee et al., 2012; Nencioni et al., 2018; Raffaghello et al., 2008). Insulin, glucose, and insulin-like growth factor 1 (IGF-1) help mediate cell growth, cell reproduction, and prevent cell death (Di Biase & Longo, 2016; Lee et al., 2012; Nencioni et al., 2018; Raffaghello et al., 2008). A measurable deficit in these hormones deprives cells of an optimal developmental environment, triggering a stress response that causes a transition from proliferation to maintenance and repair (Di Biase & Longo, 2016; Lee et al., 2012; Nencioni et al., 2018; Raffaghello et al., 2008). Healthy cells are easily able to undergo this protective response while cancer cells lack the same capability, making cancer cells more sensitive to the effects of chemotherapy (Di Biase & Longo, 2016; Lee et al., 2012; Nencioni et al., 2018; Raffaghello et al., 2008). Healthy cells also use mitochondrial oxidative phosphorylation for metabolism while cancer cells use aerobic glycolysis, also known as the Warburg effect (Liberti & Locasale, 2016; Nencioni et al., 2018). Cancer cell metabolism increases the uptake of glucose and lactate production for growth and proliferation (Liberti & Locasale, 2016; Warburg, 1956). Current research related to fasting has hypothesized that a more robust response to chemotherapy may occur with the potential for better outcomes as cancer cells are in a vulnerable state (Arends et al., 2017a; Caffa et al., 2015; Laviano & Rossi Fanelli, 2012; Lee et al., 2012; Raffaghello et al., 2008; Safdie et al., 2012). Thus, suggesting the impact of fasting on endocrine parameters could possibly lead to greater treatment response with fewer treatment side effects.
Glucose. Glucose levels among participants were reported in two studies with conflicting results. de Groot and colleagues (2020) found a statistically significant decrease in glucose among participants compliant with fasting compared with controls following a regular diet before the first cycle of chemotherapy (p = .006), as well as during the middle of therapy (p = .042). Dorff and colleagues (2016) reported no significant difference in glucose levels during fasting (p = .13).
Insulin. Hyperinsulinemia enhances tumor cell proliferation and may also contribute to tumor progression (Boyd, 2003). Three studies described decreased insulin levels among participants. de Groot and colleagues (2020) reported a statistically significant decrease in insulin among participants compliant with fasting compared with regular diet before the first cycle of chemotherapy (p = .001), as well as during the middle of therapy (p < .001). Zorn and colleagues (2018) documented a statistically significant reduction in mean insulin during fasting compared with normal diet (p < .001). Dorff and colleagues (2016) reported lower insulin levels among participants after fasting (56% after 24-hour fasting, 27% after 48-hour fasting, and 42% after 72-hour fasting). However, this decrease was not statistically significant (Dorff et al., 2016).
IGF-1. Circulating levels of IGF have been associated with cancer risk and cell proliferation leading to cancer infiltration and metastasis (Weroha & Haluska, 2012). Three studies remarked on IGF-1 levels among participants with varying results. de Groot and colleagues (2020) found a statistically significant decrease in IGF-1 among participants compliant with fasting compared with regular diet during the middle of therapy (p = .025). Zorn and colleagues (2018) documented a statistically significant reduction in mean IGF-1 level during fasting compared with normal diet (p < .001). Dorff and colleagues (2016) reported lower IGF-1 levels among participants after the first fasting period with chemotherapy. The authors noted a decrease of 30% after 24-hour fasting, 33% after 48-hour fasting, and 8% after 72-hour fasting (Dorff et al., 2016). However, the decreases in this study were not statistically significant (Dorff et al., 2016).
Quality-of-Life Measures
Chemotherapy is associated with acute and long-term side effects that may negatively impact QOL. Current research hypothesizes that intermittent fasting may reduce the side effects of chemotherapy, potentially decreasing the negative impact on QOL (Nencioni et al., 2018; Tinsley & La Bounty, 2015). Quality of life is measured using a variety of standardized questionnaires and scales (Brandberg et al., 2020; Hall et al., 2014; Hyland & Sodergren, 1996; Lugtenberg et al., 2021; Yellen et al., 1997).
Overall QOL. Five studies used multiple questionnaires and scales to measure overall QOL, reporting differing outcomes. Riedinger and colleagues (2020) noted improved QOL scores among fasting participants. Specifically, there was a statistically significant improvement of QOL scores after treatment among fasting participants compared with normal diet participants (p = .015; Riedinger et al., 2020). Quality of life was assessed using the Functional Assessment of Chronic Illness Therapy (FACIT) questionnaire (NCCN-FACT FOSI-18; Riedinger et al., 2020). Bauersfeld and colleagues (2018) documented a statistically significant improvement in QOL after chemotherapy among participants who fasted cycles 1 to 3 and then consumed normal diet for cycles 4 to 6 (p = .041). In contrast, there was not a statistically significant change in QOL measured among participants who consumed a normal diet for cycles 1 to 3 and then fasted for cycles 4 to 6 (Bauersfeld et al., 2018). Quality of life was evaluated using the Functional Assessment of Cancer Therapy-General (FACT-G) scale (Bauersfeld et al., 2018). Zorn and colleagues (2018) found no statistically significant difference in QOL between fasting participants and normal diet participants using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire. de Groot and colleagues (2020) and Lugtenberg and colleagues (2021) both analyzed data from the same clinical trial, reporting no statistically significant difference in overall QOL between fasting participants and normal diet participants using the EORTC QLQ-C30 questionnaire (de Groot et al., 2020; Lugtenberg et al., 2021).
Fatigue. The most common side effect of cancer treatment is fatigue, with clinically significant levels of fatigue negatively impacting patient survival (Mo et al., 2021; Prue et al., 2006). Two studies included in this review applied the FACIT-Fatigue questionnaire to investigate the impact of fasting on fatigue. The results were conflicting in that Bauersfeld and colleagues (2018) documented a statistically significant improvement in fatigue after chemotherapy among participants who fasted for cycles 1 to 3 and then consumed normal diet for cycles 4 to 6 (p = .006). However, there was no significant change in fatigue measured among participants who consumed normal diet for cycles 1 to 3 and then fasted for cycles 4 to 6 (p = .521; Bauersfeld et al., 2018). Additionally, Zorn and colleagues (2018) found no statistically significant difference in fatigue between fasting participants and normal diet participants using the FACIT-Fatigue questionnaire (p = .882).
Cancer Outcomes
Riedinger and colleagues (2020), de Groot and colleagues (2020), and Dorff and colleagues (2016) documented the effect of fasting during chemotherapy on cancer outcomes with contradictory findings.
Treatment Outcome. Riedinger and colleagues (2020) found no statistically significant difference in partial treatment response (p = .35) or complete treatment response (p = 1.0) between fasting participants and regular diet participants. Measurement tools used for response evaluation and criteria were not reported.
Radiographic and Pathologic Response. de Groot and colleagues (2020) and Dorff and colleagues (2016) both examined radiologic and pathologic response in participants. de Groot and colleagues (2020) documented a partial or complete pathologic response more often among fasting participants compared with regular diet participants (odds ratio [OR], 4.109, p = .016). Pathologic response was measured using the Miller and Payne score (de Groot et al., 2020). A complete or partial radiographic response was also noted more often among fasting participants compared with normal diet participants (OR, 3.168, p = .039; de Groot et al., 2020). Radiographic response was measured using Response Evaluation Criteria in Solid Tumors (RECIST; de Groot et al., 2020). Dorff and colleagues (2016) also used the RECIST method to measure radiographic response among fasting participants: eight (40%) had a partial or complete radiologic response, three (15%) had stable disease, and one (5%) had progressive disease. The RECIST method was not applied to eight (40%) fasting participants since they were treated in the adjuvant setting (Dorff et al., 2016).
Discussion
This review sought to understand the impact of fasting on patients with cancer undergoing systematic therapy. Based on a review of the existing literature, several factors influence the efficacy and applicability of fasting as a nutritional intervention. Overall compliance was adequate, particularly with fewer chemotherapy cycles, as there was a tendency for compliance to decrease over consecutive cycles of chemotherapy (Bauersfeld et al., 2018; de Groot et al., 2020; Dorff et al., 2016; Lugtenberg et al., 2021; Riedinger et al., 2020; Tang et al., 2021; Zorn et al., 2018). Weight loss was minimal and malnutrition was not identified in any participants (Bauersfeld et al., 2018; Dorff et al., 2016; Lugtenberg et al., 2021; Riedinger et al., 2020; Tang et al., 2021; Zorn et al., 2018). However, it should be noted only participants with normal weight were included in the studies and no data are available related to participants identified as underweight at baseline. Fasting appears to be well tolerated, with several studies reporting no to low occurrence of severe chemotherapy-induced toxicities (Bauersfeld et al., 2018; de Groot et al., 2020; Dorff et al., 2016; Riedinger et al., 2020; Zorn et al., 2018). There were no serious side effects reported in the seven studies included in this review, as well as no documented deaths related to fasting. There were fewer chemotherapy dose reductions or cycle delays among fasting participants, indicating the potential for better chemotherapy tolerance (Bauersfeld et al., 2018; Riedinger et al., 2020; Zorn et al., 2018). In two studies, there was a trend toward a partial or complete radiologic and/or pathologic response among the majority of fasting participants (de Groot et al., 2020; Dorff et al., 2016). Given the data were only collected in two studies, additional research is needed to confirm the trend in radiographic and pathologic outcomes.
There were conflicting results reported for endocrine parameters. While all three studies examining endocrine parameters utilized a fasting period greater than 48 hours, the differences in fasting duration among the study designs may account for the contradictory results. de Groot and colleagues (2020) and Zorn and colleagues (2018) required a longer fasting period with a 96-hour fast, while Dorff and colleagues (2016) used a 72-hour fast, leading to speculation that the longer fasting period used prior to the initiation of chemotherapy by de Groot and colleagues (2020) and Zorn and colleagues (2018) potentially allowed for a greater measurable metabolic response at the cellular level. One study (de Groot et al., 2020) found fasting decreased glucose by statistically significant values, while another study (Dorff et al., 2016) did not find a significant difference. Two studies (de Groot et al., 2020; Zorn et al., 2018) documented a statistically significant decrease in insulin among fasting participants, while one study (Dorff et al., 2016) noted no difference in insulin levels with fasting. Two studies (de Groot et al., 2020; Zorn et al., 2018) reported a statistically significant decrease in IGF-1 among fasting participants, while one study (Dorff et al., 2016) showed a trend toward lower IGF-1 levels with fasting that was not statistically significant.
Additionally, there were conflicting results documented for QOL and fatigue between studies, making the relevance of the results inconclusive. A variety of instruments to evaluate QOL and fatigue were utilized between the studies, which could allow for bias and may explain the differing results. Two studies (de Groot et al., 2020; Lugtenberg et al., 2021) that analyzed the same data reported no significant improvement in QOL scores after fasting; however, this could also be construed as positive in that a fasting intervention did not alter QOL. It was also notable that in the de Groot and colleagues (2020) and Lugtenberg and colleagues (2021) studies, normal diet participants were prescribed dexamethasone during chemotherapy to reduce side effects allowing for the possibility that measurable differences between the fasting and normal diet groups were masked by dexamethasone in the normal diet group. One study (Bauersfeld et al., 2018) found fasting significantly improved fatigue, while another study (Zorn et al., 2018) found no difference in levels of fatigue with fasting.
While intermittent fasting during systemic therapy appears to be safe and feasible among normal weight patients with cancer and has promising potential for improved outcomes, there is not enough evidence to widely support its use in the clinical setting. Further exploration as to this avenue of dietary modification may be warranted as the current findings do not indicate an adverse impact to this approach. However, based on the current evidence, patients with cancer should only consider intermittent fasting as an adjunct to systemic therapy if they are participating in a research study or under the approval of their provider. Patients who are underweight should avoid fasting as there is no evidence that it is safe in this population.
Limitations
This review has several limitations which should caution practitioners from recommending intermittent fasting to patients receiving systemic therapy. To date, there is minimal evidence regarding intermittent fasting as a diet modification in the cancer population; only seven studies were identified that met the established inclusion and exclusion criteria for this review. The low volume of studies offers limited insight into the impact of fasting on patients with cancer. However, it is worth noting the seven studies included have strong levels of evidence (Burns et al., 2011): three are randomized control trials, one is a randomized crossover trial, one is a control crossover trial, and two are prospective cohort studies. However, it is notable that although statistically powered, the study samples were small with all but two having fewer than 50 participants. A gender disparity also existed among all of the studies, with the study populations consisting of 15 males and 127 females, thus further limiting the generalizability of the findings. Race/ethnicity was not recorded in several of the studies, making it difficult to generalize the results to a diverse population. Additionally, several of the studies were conducted in specific geographical locations with homogeneous populations, further limiting the generalizability of the study findings.
It should also be noted that the majority of participants in these studies had breast or gynecologic cancers, creating an unintentional bias toward cancer type, therapy class, and highlighting a study population that is overwhelmingly female by default. Cancer stage was not documented in every study. Therefore, it is unclear if there is a difference in the impact of fasting on patients receiving systemic therapy for primary tumors (neoadjuvant or adjuvant) compared with metastatic disease. Each study used a different variation of intermittent fasting with a wide spectrum of approved calorie consumption and differing fasting schedules. This makes it difficult to compare data and identify the most beneficial nutritional intervention for patients with cancer receiving systemic therapy. Long-term monitoring follow-up periods would also be beneficial for analyzing the potential for long-term, sustained benefits of fasting on cancer outcomes and survival data.
Conclusion
Patients with cancer often seek practices to positively impact their treatment outcomes, disease status, and QOL through nutrition. There is growing interest surrounding intermittent fasting as an adjunct to chemotherapy. However, there is limited research to support the use of intermittent fasting during systemic therapy as a safe, feasible, and impactful intervention for patients with cancer. To the authors’ knowledge, this is the first peer-reviewed literature review that examines the current evidence specific to patients choosing an intermittent fasting regimen while undergoing chemotherapy or chemotherapy in conjunction with immunotherapy. Data from the seven research articles included in this review suggest that intermittent fasting has the potential to positively impact cancer treatment, although additional larger randomized controlled trials are needed to validate these early findings and further explore the impact of fasting on systemic therapy.
Disclosure
The authors have no conflicts of interest to disclose.
References
Arends, J., Bachmann, P., Baracos, V., Barthelemy, N., Bertz, H., Bozzetti, F.,...Preiser, J. C. (2017a). ESPEN guidelines on nutrition in cancer patients. Clinical Nutrition, 36(1), 11–48. https://doi.org/10.1016/j.clnu.2016.07.015
Arends, J., Baracos, V., Bertz, H., Bozzetti, F., Calder, P. C., Deutz, N. E. P.,...Weimann, A. (2017b). ESPEN expert group recommendations for action against cancer-related malnutrition. Clinical Nutrition, 36(5), 1187–1196. https://doi.org/10.1016/j.clnu.2017.06.017
Bauersfeld, S. P., Kessler, C. S., Wischnewsky, M., Jaensch, A., Steckhan, N., Stange, R.,...Michalsen, A. (2018). The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: A randomized cross-over pilot study. BMC Cancer, 18(1), 476. https://doi.org/10.1186/s12885-018-4353-2
Boyd, D. B. (2003). Insulin and cancer. Integrative Cancer Therapies, 2(4), 315–329. https://doi.org/10.1177/1534735403259152
Bozzetti, F., Mariani, L., Lo Vullo, S., Amerio, M. L., Biffi, R., Caccialanza, G.,...Vigevani, E. (2012). The nutritional risk in oncology: A study of 1,453 cancer outpatients. Supportive Care in Cancer, 20(8), 1919–1928. https://doi-org.foyer.swmed.edu/10.1007/s00520-012-1387-x
Brandberg, Y., Johansson, H., Hellström, M., Gnant, M., Möbus, V., Greil, R.,...Bergh, J. (2020). Long-term (up to 16 months) health-related quality of life after adjuvant tailored dose-dense chemotherapy vs. standard three-weekly chemotherapy in women with high-risk early breast cancer. Breast Cancer Research & Treatment, 181(1), 87–96. https://doi.org/10.1007/s10549-020-05602-9
Brandhorst, S., Choi, I. Y., Wei, M., Cheng, C. W., Sedrakyan, S., Navarrete, G.,...Longo, V. D. (2015). A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metabolism, 22(1), 86–99. https://doi.org/10.1016/j.cmet.2015.05.012
Brown, D. (2020). A review of the PubMed PICO tool: Using evidence-based practice in health education. Health Promotion Practice, 21(4), 496–498. https://doi.org/10.1177/1524839919893361
Burns, P. B., Rohrich, R. J., & Chung, K. C. (2011). The levels of evidence and their role in evidence-based medicine. Plastic & Reconstructive Surgery, 128(1), 305–310. https://doi.org/10.1097/PRS.0b013e318219c171
Caffa, I., Longo, V. D., & Nencioni, A. (2015). Fasting plus tyrosine kinase inhibitors in cancer. Aging, 7(12), 1026–1027. https://doi.org/10.18632/aging.100857
Cleeland, C. S., Allen, J. D., Roberts, S. A., Brell, J. M., Giralt, S. A., Khakoo, A. Y.,...Skillings, J. (2012). Reducing the toxicity of cancer therapy: Recognizing needs, taking action. Nature Reviews Clinical Oncology, 9(8), 471–478. https://doi.org/10.1038/nrclinonc.2012.99
D’Aronzo, M., Vinciguerra, M., Mazza, T., Panebianco, C., Saracino, C., Pereira, S. P.,...Pazienza, V. (2015). Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget, 6(21), 18545–18557. https://doi.org/10.18632/oncotarget.4186
de Groot, S., Lugtenberg, R. T., Cohen, D., Welters, M. J. P., Ehsan, I., Vreeswijk, M. P. G.,...Dutch Breast Cancer Research Group (BOOG). (2020). Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nature Communications, 11(1), 3083. https://doi.org/10.1038/s41467-020-16138-3
Di Biase, S., & Longo, V. D. (2016). Fasting-induced differential stress sensitization in cancer treatment. Molecular & Cellular Oncology, 3(3), e1117701. https://doi.org/10.1080/23723556.2015.1117701
Dirks, A. J., & Leeuwenburgh, C. (2006). Caloric restriction in humans: Potential pitfalls and health concerns. Mechanisms of Ageing and Development, 127(1), 1–7. https://doi.org/10.1016/j.mad.2005.09.001
Dorff, T. B., Groshen, S., Garcia, A., Shah, M., Tsao-Wei, D., Pham, H.,...Quinn, D. I. (2016). Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer, 16, 360. https://doi.org/10.1186/s12885-016-2370-6
Hall, E., Cameron, D., Waters, R., Barrett-Lee, P., Ellis, P., Russell, S.,...Hopwood, P. (2014). Comparison of patient reported quality of life and impact of treatment side effects experienced with a taxane-containing regimen and standard anthracycline based chemotherapy for early breast cancer: 6-year results from the UK TACT trial (CRUK/01/001). European Journal of Cancer, 50(14), 2375–2389. https://doi.org/10.1016/j.ejca.2014.06.007
Hébuterne, X., Lemarié, E., Michallet, M., de Montreuil, C. B., Schneider, S. M., & Goldwasser, F. (2014). Prevalence of malnutrition and current use of nutrition support in patients with cancer. Journal of Parenteral and Enteral Nutrition, 38(2), 196–204. https://doi.org/10.1177/0148607113502674
Hyland, M. E., & Sodergren, S. C. (1996). Development of a new type of global quality of life scale, and comparison of performance and preference for 12 global scales. Quality of Life Research, 5(5), 469–480. https://doi.org/10.1007/bf00540019
International Food Information Council. (2021). 2021 Food & Health Survey. Food Insight. https://foodinsight.org/2021-food-health-survey/
Kerndt, P. R., Naughton, J. L., Driscoll, C. E., & Loxterkamp, D. A. (1982). Fasting: The history, pathophysiology and complications. Western Journal of Medicine, 137(5), 379–399. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1274154/
Ketelslegers, J. M., Maiter, D., Maes, M., Underwood, L. E., & Thissen, J. P. (1996). Nutritional regulation of the growth hormone and insulin-like growth factor-binding proteins. Hormone Research in Paediatrics, 45(3–5), 252–257. https://doi.org/10.1159/000184797
Klement, R. J., & Champ, C. E. (2014). Calories, carbohydrates, and cancer therapy with radiation: exploiting the five R’s through dietary manipulation. Cancer Metastasis Reviews, 33(1), 217–229. https://doi.org/10.1007/s10555-014-9495-3
Laviano, A., & Rossi Fanelli, F. (2012). Toxicity in chemotherapy--when less is more. New England Journal of Medicine, 366(24), 2319–2320. https://doi.org/10.1056/NEJMcibr1202395
Lee, C., & Longo, V. D. (2011). Fasting vs dietary restriction in cellular protection and cancer treatment: From model organisms to patients. Oncogene, 30(30), 3305–3316. https://doi.org/10.1038/onc.2011.91
Lee, C., Raffaghello, L., Brandhorst, S., Safdie, F. M., Bianchi, G., Martin-Montalvo, A.,...Longo, V. D. (2012). Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Science Translational Medicine, 4(124), 124ra127. https://doi.org/10.1126/scitranslmed.3003293
Lee, S. F., Wyld, D., Brown, T., & Eastgate, M. A. (2018). Dietary patterns and attitudes in cancer patients. Journal of Clinical Oncology, 36(15_suppl), e22055–e22055. https://doi.org/10.1200/JCO.2018.36.15_suppl.e22055
Liberti, M. V., & Locasale, J. W. (2016). The Warburg Effect: How does it benefit cancer cells? Trends in Biochemical Sciences, 41(3), 211–218. https://doi.org/10.1016/j.tibs.2015.12.001
Lo Re, O., Panebianco, C., Porto, S., Cervi, C., Rappa, F., Di Biase, S.,...Vinciguerra, M. (2018). Fasting inhibits hepatic stellate cells activation and potentiates anti-cancer activity of Sorafenib in hepatocellular cancer cells. Journal of Cellular Physiology, 233(2), 1202–1212. https://doi.org/10.1002/jcp.25987
Longo, V. D., & Mattson, M. P. (2014). Fasting: Molecular mechanisms and clinical applications. Cell Metabolism, 19(2), 181–192. https://doi.org/10.1016/j.cmet.2013.12.008
Lugtenberg, R. T., de Groot, S., Kaptein, A. A., Fischer, M. J., Kranenbarg, E. M., Carpentier, M. D.,...Dutch Breast Cancer Research Group (BOOG) (2021). Quality of life and illness perceptions in patients with breast cancer using a fasting mimicking diet as an adjunct to neoadjuvant chemotherapy in the phase 2 DIRECT (BOOG 2013-14) trial. Breast Cancer Research & Treatment, 185(3), 741–758. https://doi.org/https://dx.doi.org/10.1007/s10549-020-05991-x
Mansell, P. I., & Macdonald, I. A. (1990). The effect of starvation on insulin-induced glucose disposal and thermogenesis in humans. Metabolism: Clinical & Experimental, 39(5), 502–510. https://doi.org/10.1016/0026-0495(90)90009-2
Mas, S., Le Bonniec, A., & Cousson-Gélie, F. (2019). Why do women fast during breast cancer chemotherapy? A qualitative study of the patient experience. British Journal of Health Psychology, 24(2), 381–395. https://doi.org/https://doi.org/10.1111/bjhp.12358
Mo, J., Darke, A. K., Guthrie, K. A., Sloan, J. A., Unger, J. M., Hershman, D. L.,...Krouse, R. S. (2021). Association of fatigue and outcomes in advanced cancer: An analysis of four SWOG treatment trials. JCO Oncology Practice, 17(8), e1246–e1257. https://doi.org/10.1200/op.20.01096
Muscaritoli, M., Arends, J., Bachmann, P., Baracos, V., Barthelemy, N., Bertz, H.,...Bischoff, S. C. (2021). ESPEN practical guideline: Clinical nutrition in cancer. Clinical Nutrition, 40(5), 2898–2913. https://doi.org/10.1016/j.clnu.2021.02.005
National Cancer Institute. (2022). Common terminology criteria for adverse events (CTCAE). CTEP Cancer Therapy Evaluation Program. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
Naveed, S., Aslam, M., & Ahmad, A. (2014). Starvation based differential chemotherapy: A novel approach for cancer treatment. Oman Medical Journal, 29(6), 391–398. https://doi.org/10.5001/omj.2014.107
Nencioni, A., Caffa, I., Cortellino, S., & Longo, V. D. (2018). Fasting and cancer: Molecular mechanisms and clinical application. Nature Reviews. Cancer, 18(11), 707–719. https://doi.org/10.1038/s41568-018-0061-0
Omodei, D., & Fontana, L. (2011). Calorie restriction and prevention of age-associated chronic disease. FEBS Lett, 585(11), 1537–1542. https://doi.org/10.1016/j.febslet.2011.03.015
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D.,...Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. https://doi.org/10.1136/bmj.n71
Panebianco, C., Adamberg, K., Adamberg, S., Saracino, C., Jaagura, M., Kolk, K.,...Pazienza, V. (2017). Engineered resistant-starch (ERS) diet shapes colon microbiota profile in parallel with the retardation of tumor growth in in vitro and in vivo pancreatic cancer models. Nutrients, 9(4), 331. https://doi.org/10.3390/nu9040331
Prue, G., Rankin, J., Allen, J., Gracey, J., & Cramp, F. (2006). Cancer-related fatigue: A critical appraisal. European Journal of Cancer, 42(7), 846–863. https://doi.org/10.1016/j.ejca.2005.11.026
Raffaghello, L., Lee, C., Safdie, F. M., Wei, M., Madia, F., Bianchi, G., & Longo, V. D. (2008). Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proceedings of the National Academy of Sciences, 105(24), 8215–8220. https://doi.org/10.1073/pnas.0708100105
Riedinger, C. J., Kimball, K. J., Kilgore, L. C., Bell, C. W., Heidel, R. E., & Boone, J. D. (2020). Water only fasting and its effect on chemotherapy administration in gynecologic malignancies. Gynecologic Oncology, 159(3), 799–803. https://doi.org/10.1016/j.ygyno.2020.09.008
Romijn, J. A., Godfried, M. H., Hommes, M. J., Endert, E., & Sauerwein, H. P. (1990). Decreased glucose oxidation during short-term starvation. Metabolism: Clinical & Experimental, 39(5), 525–530. https://doi.org/10.1016/0026-0495(90)90012-2
Safdie, F., Brandhorst, S., Wei, M., Wang, W., Lee, C., Hwang, S.,...Longo, V. D. (2012). Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS ONE, 7(9), e44603. https://doi.org/10.1371/journal.pone.0044603
Sullivan, E. S., Rice, N., Kingston, E., Kelly, A., Reynolds, J. V., Feighan, J.,...Ryan, A. M. (2021). A national survey of oncology survivors examining nutrition attitudes, problems and behaviours, and access to dietetic care throughout the cancer journey. Clinical Nutrition ESPEN, 41, 331–339. https://doi.org/10.1016/j.clnesp.2020.10.023
Tang, C. C., Huang, T. C., Tien, F. M., Lin, J. M., Yeh, Y. C., & Lee, C. Y. (2021). Safety, feasibility, and effects of short-term calorie reduction during induction chemotherapy in patients with diffuse large b-cell lymphoma: A pilot study. Nutrients, 13(9). https://doi.org/http://dx.doi.org/10.3390/nu13093268
Tinsley, G. M., & La Bounty, P. M. (2015). Effects of intermittent fasting on body composition and clinical health markers in humans. Nutrition Reviews, 73(10), 661–674. https://doi.org/10.1093/nutrit/nuv041
Warburg, O. (1956). On the origin of cancer cells. Science, 123(3191), 309–314. https://doi.org/10.1126/science.123.3191.309
Weroha, S. J., & Haluska, P. (2012). The insulin-like growth factor system in cancer. Endocrinology and Metabolism Clinics of North America, 41(2), 335–350. https://doi.org/10.1016/j.ecl.2012.04.014
Yellen, S. B., Cella, D. F., Webster, K., Blendowski, C., & Kaplan, E. (1997). Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain and Symptom Management, 13(2), 63–74. https://doi.org/10.1016/s0885-3924(96)00274-6
Zick, S. M., Snyder, D., & Abrams, D. I. (2018). Pros and cons of dietary strategies popular among cancer patients. Oncology (Williston Park), 32(11), 542–547.
Zorn, S., Raynor, A., Urbain, P., Schauble, R., Mihailescu, M., & Bertz, H. (2018). Impact of short-term modified fasting and the combination with a fasting supportive diet during chemotherapy on the incidence and severity of chemotherapy-induced toxicities in cancer patients - a randomised controlled cross-over pilot study (MOFAX). Aktuelle Ernahrungsmedizin, 43(3), 244–245. https://doi.org/10.1055/s-0038-1647234