Research and Scholarship
Clinical Utility of the 31-Gene Expression Profile Test on the Management of Cutaneous Melanoma by Nurse Practitioners and Physician Assistants
Renata Block,(1) MMS, PA-C, Deborah Patterson,(2) MMS, PA-C, Jennifer J. Siegel,(2) PhD, Brian Martin,(2) PhD, Ann P. Quick,(2) PhD, and Jillian Hunt,(3) MSN, APRN, FNP-C, AOCNP®
From (1)Advanced Dermatology & Aesthetic Medicine, Chicago, Illinois; (2)Castle Biosciences, Inc., Friendswood, Texas; (3)Cincinnati Cancer Advisors, Cincinnati, Ohio
Authors’ disclosures of conflicts of interest are found at the end of this article.
Correspondence to: Brian Martin, PhD,
Castle Bioscience, Inc., 505 S. Friendswood Dr., Friendswood, TX 77546
E-mail: bmartin@castlebiosciences.com
J Adv Pract Oncol 2023;14(7):586–596 |
https://doi.org/10.6004/jadpro.2023.14.7.3 |
© 2023 BroadcastMed LLC

 ABSTRACT
ABSTRACT
Objective: The 31-gene expression profile (31-GEP) can predict the risk of recurrence and metastasis in cutaneous melanoma (CM). We assessed the viewpoints and use of 31-GEP testing by physician assistants (PAs) and nurse practitioners (NPs) for patients with CM. Methods: NPs and PAs (n = 369) completed an 18-question online survey about their viewpoints and use of the 31-GEP risk-stratification test. Results: Most practitioners (n = 334, 90.5%) felt prognostic testing improved patient care and would recommend the 31-GEP to a colleague (n = 333, 90.2%) or a friend or family member (n = 289, 78.3%) who was diagnosed with CM. The 31-GEP test was used by 176 respondents in the preceding 12 months (53%). Among users of the 31-GEP test, 78% stated that the results would impact follow-up schedule and referral, 66% overall treatment decisions, 62% sentinel lymph node biopsy recommendations, and 50% surveillance imaging. In thin tumors (″ 1 mm), 82% of 31-GEP users and 44% of nonusers stated that the 31-GEP results would impact their treatment plan decisions. Conclusion: The 31-GEP test significantly impacts treatment plans in CM, particularly for thin and stage I melanomas. Importantly, even nonusers stated that 31-GEP test results would impact treatment plans as well as recommendations to a friend or family member.
ARTICLE
Cutaneous melanoma (CM) is the fifth most common malignancy in the United States, resulting in over 7,000 deaths annually (National Cancer Institute, 2023). The majority of patients with CM classified as low risk (stage I or II) according to American Joint Committee on Cancer (AJCC) staging guidelines (Gershenwald et al., 2017) have an excellent prognosis. However, due to the large absolute number of patients diagnosed with early-stage disease, many will have disease recurrence or death each year (Morton et al., 2014). Therefore, it is important to develop additional prognostic testing methods that can more accurately stratify patient risk, identifying high-risk patients necessitating imaging and/or therapeutic intervention and conversely, those who are indeed at low risk of disease progression.
Molecular-based technologies for predicting outcomes have received increasing interest and are commonly used in breast cancer, uveal melanoma, and other diseases to improve patient care (Sun et al., 2021; Hijazo-Pechero et al., 2021; Field & Harbour, 2014). Moreover, there is growing interest in molecular risk stratification in CM using gene expression tests that provide prognostic information (Cohen & Kurzrock, 2022; Bollard et al., 2021), including the 31-gene expression profile test (31-GEP; Castle Biosciences, Inc., Friendswood, Texas), which provides prognostic information independent of other clinical and pathological factors by analyzing differential gene expression of a validated panel of 31 gene targets (Gerami et al., 2015a, 2015b). The 31-GEP provides a continuous probability score between zero and one to stratify a patient’s risk of recurrence or metastasis. Results are classified as low risk (Class 1A), intermediate risk (Class 1B and 2A), or high risk (Class 2B) for tumor recurrence or metastasis. The accuracy of 31-GEP testing in patients with stage I to III CM has been validated in several prospective multicenter studies (Lawson et al., 2015; Ferris et al., 2017; Dillon et al., 2018; Greenhaw et al., 2018; Keller et al., 2019; Podlipnik et al., 2019; Hsueh et al., 2021).
Multiple clinical utility studies have shown that providers use 31-GEP test results to help guide treatment management and surveillance plans (Dillon et al., 2018; Berger et al., 2016; Farberg et al., 2017; Schuitevoerder et al., 2018; Mirsky et al., 2018); however, because the care of early-stage skin cancer is largely diagnosed and managed in private dermatology practices, which include dermatologists as well as nurse practitioners (NPs) and physician assistants (PAs), it is important to identify how various front-line clinicians engage with the 31-GEP test. Worldwide, the number of advanced practice providers, including PAs and NPs, is expected to continue increasing, and they will continue to play an integral role in delivering quality oncology patient care (Bruinooge et al., 2018; Ross et al., 2010; Sheringham et al., 2021).
The purpose of this study was to assess the viewpoints, experience, and clinical use of 31-GEP testing by PAs and NPs for patients with CM.
Methods
Survey Study
We collected 369 responses (Table 1) from self-identified NPs or PAs to an 18-question electronic survey study that was available via a website link during the following conferences: Fall Clinical NPPA 2020 (virtual, April 3–5, 2020), Fall Clinical 2020 (virtual, October 29–November 1, 2020), and Winter Clinical 2021 (virtual, January 16–24, 2021). The survey included 18 questions regarding practice demographics, factors considered in choosing to order the 31-GEP test for their patients, integration of the 31-GEP results into clinical management, and their opinions regarding the utility of the 31-GEP test results. The WCG Institutional Review Board (IRB) approved the study and, although the IRB granted a waiver of consent, the questionnaire included a consent statement participants read before proceeding with the questionnaire. Participants were compensated with a $25 gift card for their participation. Although the study link was available in conference promotional materials, we do not have data regarding how many eligible participants were truly aware of the study. Thus, we are unable to ascertain a response rate for the study.
Statistical Analysis
Differences between survey responses were compared using Chi-squared analysis. Statistical significance was defined as p values < .05.
Results
Participant Demographics
Three hundred sixty-nine self-identified NPs and PAs who attended one of the three conferences completed the survey (Table 1). Of these, 176 (47.7%) reported using the 31-GEP test. Most of the respondents (n = 190, 51.5%) reported being in practice for 1–10 years, while only 7 (1.9%) had been in practice for over 30 years. There was no significant difference between 31-GEP users and nonusers regarding how long the provider had been in clinical practice (p = .306). Most respondents (n = 341, 92.4%) worked in private practice.
Factors That Influence Ordering 31-GEP Testing
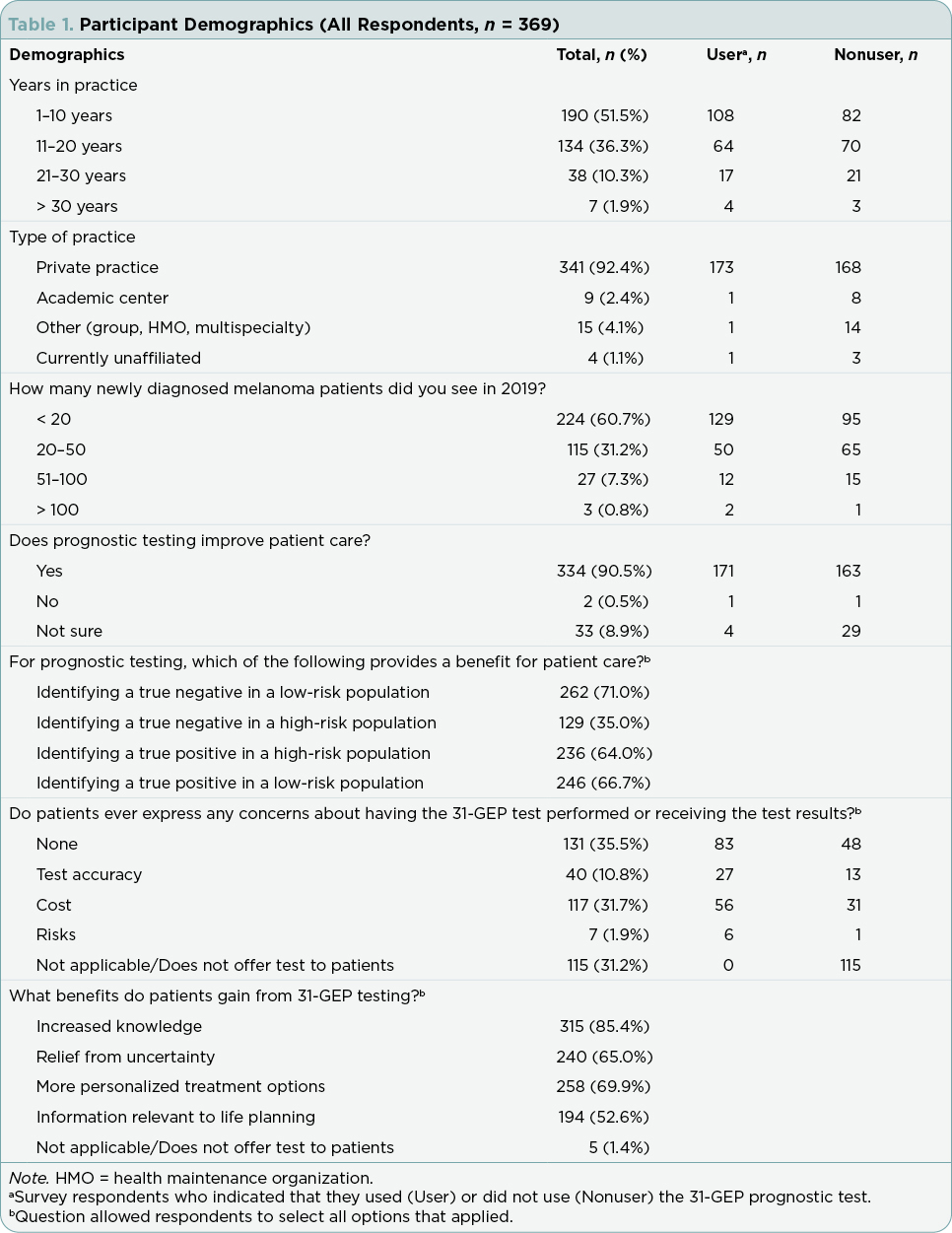
When considering ordering the 31-GEP, a majority of users considered histological subtype (n = 110, 62.5%), Clark level (n = 110, 62.5%), and patient demographics (e.g., age, sex, clinical history; n = 107, 60.8%; Figure 1A). Additionally, tumor location was a factor considered by 41.5% (n = 73) of respondents, and 10.2% (n = 18) selected none of the above. Tumor-specific factors that practitioners considered included Breslow thickness ≥ 0.8 mm (n = 156, 88.6%; Figure 1B), presence of ulceration (n = 114, 64.8%), mitotic rate (n = 101, 57.4%), and negative sentinel lymph node biopsy (SLNB; n = 45, 25.6%). Few respondents selected none of the above (n = 9, 5.1%).
Utility of 31-GEP Results in Treatment Plan
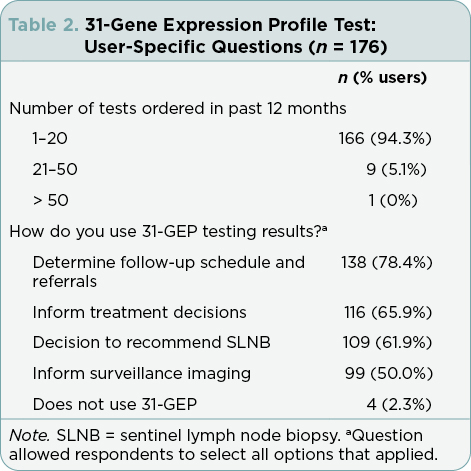
The majority of test users stated that they used the test results to determine follow-up schedules and referrals (n = 138, 78.4%), inform treatment decisions (n = 116, 65.9%), as part of the decision to recommend a patient receive or forego SLNB (n = 109, 61.9%), and inform surveillance imaging (n = 99, 50%; Table 2).
Among all respondents, most (n = 229, 62.0%) replied that a high-risk (Class 2B) result for 31-GEP testing would alter their treatment plan for a patient with a thin tumor (Figure 2A). Specifically, the majority of users (n = 145, 82.4%) and almost half of nonusers (n = 84, 43.5%, p < .001 vs. users) stated that a Class 2B result would alter their treatment plans. Significantly fewer users than nonusers (n = 26, 14.8% vs. n = 101, 52.3%, respectively, p < .001) responded that they were unsure if a Class 2B result for a thin tumor would change their treatment plans. Few nonusers or users responded that a Class 2B result would not change their treatment plans (n = 8, 4.1% of nonusers and n = 5, 2.8% of users).
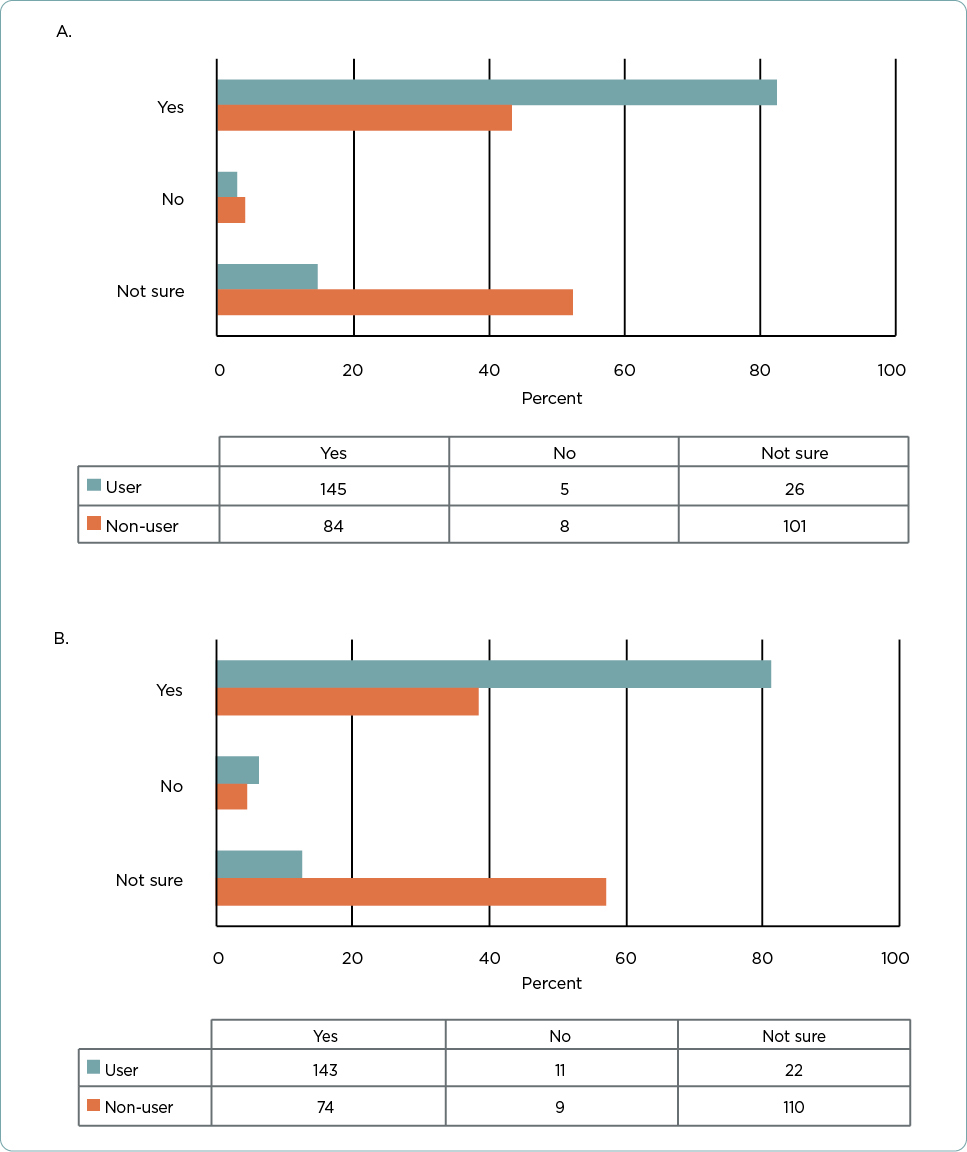
Results were similar when asked if receiving a Class 2B result for a stage I tumor would change their treatment plans (Figure 2B). More than half (n = 217, 58.8%) of all respondents stated that it would alter their treatment plans. Again, users were significantly more likely to state that a Class 2B result in a stage I tumor would change their treatment plans (n = 143, 81.3% for users vs. n = 74, 38.3% for nonusers, p < .001). Users were also significantly less likely to respond that they were unsure if a Class 2B result would change their treatment plans than nonusers (n = 22, 12.5% vs. n = 110, 57.0%, respectively, p < .001).
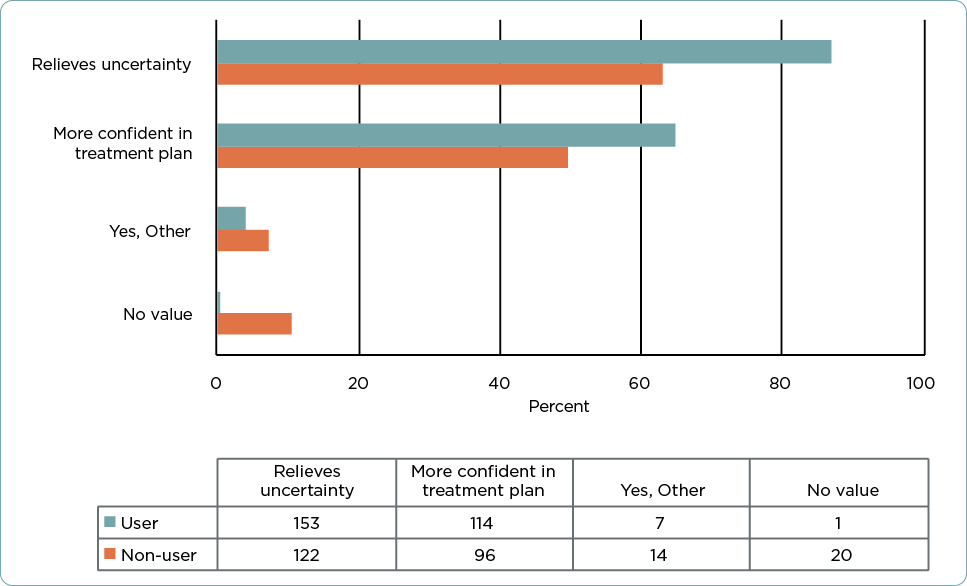
Finally, providers were asked if there was value in receiving a low-risk 31-GEP test result (Class 1A) for a patient with a T1 tumor. Both users and nonusers indicated that a Class 1A result would benefit patients by relieving uncertainty about their cancer (n = 275, 74.5%), and it benefitted providers by making them feel more confident in their chosen treatment plan (n = 210, 56.9%; Figure 3). A small number (n = 21, 5.7%), almost entirely nonusers, did not feel that the Class 1A 31-GEP test result added additional value for patients with a T1a tumor.
Viewpoints Regarding Prognostic Testing Utility
When asked, “Do you think that patient care is improved when comprehensive prognostic information is available?” the overwhelming majority of all survey respondents (n = 334, 90.5%) responded affirmatively (Table 1). Only 2 participants (0.5%) responded that they did not feel patient care was improved with prognostic testing, and the rest (n = 33, 8.9%) did not know. Nonusers were significantly more likely to indicate that they did not know if patient care was improved with prognostic testing than were users (n = 29, 15.0% vs. 4, 2.3%, respectively; p < .001).
Viewpoints Regarding 31-GEP Testing
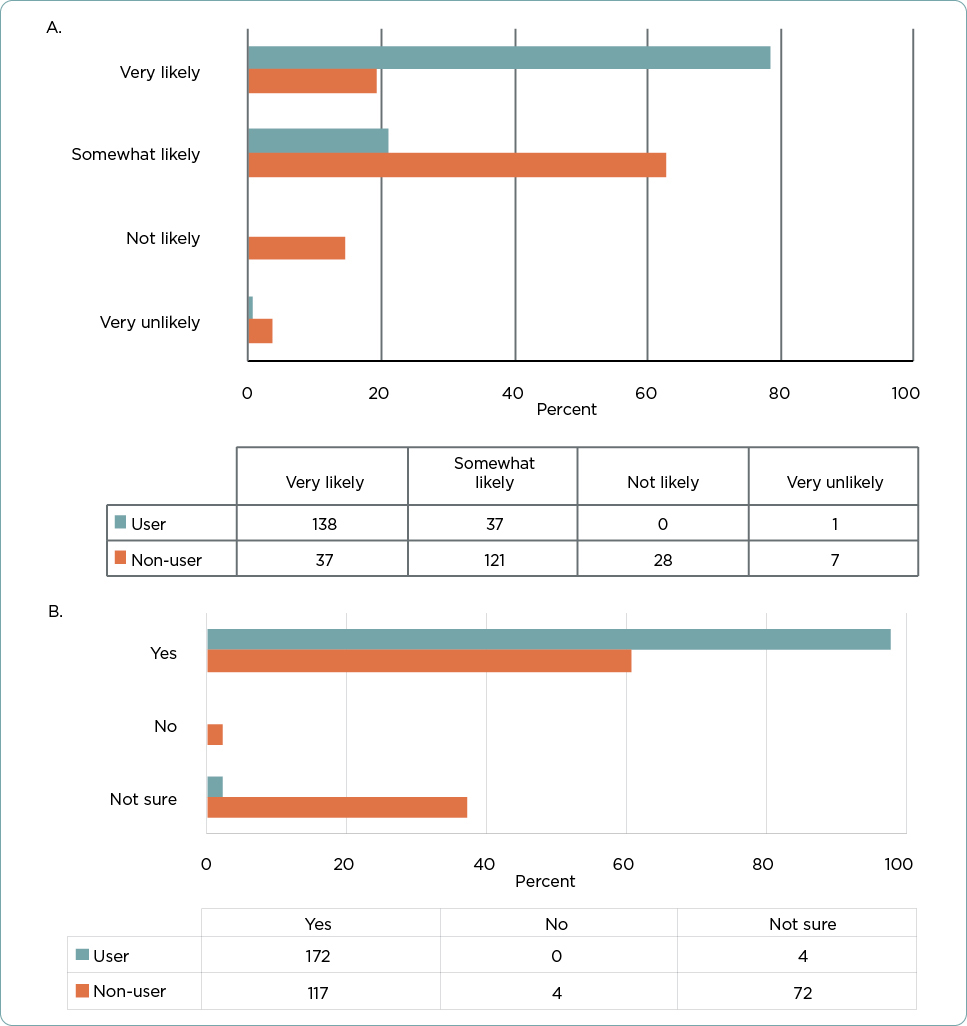
Almost all practitioners who reported using 31-GEP testing answered that they would be very or somewhat likely to recommend the test to a colleague (n = 175 out of 176, 99.4%; Figure 4A). Even among nonusers of the test, most would recommend it to a colleague (n = 158, 81.9%).
The majority of participants (n = 289, 78.3%) responded that they would recommend additional prognostic testing, such as 31-GEP testing, to a close friend or family member diagnosed with CM (Figure 4B). Again, users were significantly less likely to respond that they were not sure if they would recommend prognostic testing to a friend or relative than were non-31-GEP test users (n = 4, 2.3% vs. 72, 37.3%, respectively; p < .001); however, even the majority of nonusers (n = 117, 60.6%) stated they would recommend prognostic testing to a friend or family member. Only four respondents (all nonusers) indicated that they would not recommend prognostic testing to a friend or family member.
Discussion
Across cancer types, GEP testing has demonstrated value as a risk-stratification tool for physicians and health-care providers to guide treatment and surveillance planning for patients (Sun et al., 2021; Hijazo-Pechero et al., 2021; Cohen & Kurzrock, 2022; Farberg et al., 2017; Mirsky et al., 2018; Chandler et al., 2018; Ward et al., 2013; Xin et al., 2017). Although most patients with stage I or II tumors have a good prognosis and will not experience recurrence, many will still have poor outcomes (Gershenwald et al., 2017; Morton et al., 2014). National Comprehensive Cancer Network guidelines, while not currently endorsing the use of GEP in CM, recognize the importance of clinical judgement in selecting treatment plans for patients and recommend a patient’s care be tailored to individualized risk for poor outcomes. Previous studies have demonstrated that the 31-GEP provides clinically actionable prognostic information when combined or compared with known clinicopathologic factors (Greenhaw et al., 2018; Whitman et al., 2021; Jarell et al., 2022). The present study further establishes that practitioners utilize prognostic testing, including the 31-GEP, in the context of other clinicopathologic features to provide the most comprehensive patient care. Tools that more accurately predict which patients will experience recurrence and metastasis could help improve patient outcomes while minimizing unnecessary procedures or surveillance imaging in patients truly at low risk of recurrence. The 31-GEP testing panel has been shown to accurately predict risk of recurrence independently and in combination with AJCC staging (Greenhaw et al., 2018; Keller et al., 2019; Podlipnik et al., 2019; Hsueh et al., 2021). Given its utility as a prognostic indicator, we report the results of a survey study assessing the use and viewpoints of NPs and PAs toward the 31-GEP test.
Nurse practitioners and PAs who have incorporated 31-GEP testing into their practice report that it adds value for their patients and clinical decisions and that receiving a high-risk GEP result (Class 2B) for a patient with an otherwise lower-risk tumor would change their treatment plan for that patient. Additionally, providers feel that their patients with T1 or stage I tumors who receive a low-risk GEP result (Class 1A) benefit from increased clinician confidence in the treatment plan and their patients benefit from increased peace of mind about their prognosis.
Our study indicates that over 90% of NPs and PAs feel that providing prognostic information about a patient’s melanoma is valuable. The providers participating in our study report that prognostic information for CM allows them to make more informed treatment planning decisions, such as follow-up schedules and referrals for SLNB. These positions are echoed by physicians, as previously published survey results of dermatologists, medical oncologists, and surgical oncologists indicated similar viewpoints about prognostic testing and its utility in their clinical practices (Marson et al., 2021). Additionally, most NPs and PAs, even those who did not report current use of 31-GEP testing, would recommend prognostic testing to their colleague or to someone they know if they were diagnosed with CM.
Intriguingly, most nonusers of the 31-GEP test would recommend it to a colleague. A potential reason for this may be that the survey asked about 31-GEP test use in the prior 12 months; therefore, it is possible that some respondents who used 31-GEP testing more than 12 months previously would have been considered nonusers but may have previously used 31-GEP testing. Additionally, because most NPs and PAs in our study work in a larger private practice, some of the respondents may personally desire to order prognostic testing for their patients, but the testing may not yet be part of the standard-of-care treatment in the practice they work.
This study was conducted at three dermatology-focused conferences to focus on providers working in practices likely to be involved in patients’ melanoma diagnoses. Most respondents worked in a private practice setting, and therefore their responses may not be representative of those practitioners who work in other settings. Additionally, providers who work in other fields, such as oncology or family practice, that also diagnose melanoma may not be represented.
Conclusion
Providers believe prognostic testing is important and adds value to patient care and decision-making. Users of the 31-GEP test for CM report that it can help them provide more directed and personalized care to patients with CM and would recommend the test to their colleagues.
Disclosure
This study was funded by Castle Biosciences, Inc. Ms. Block has no conflicts of interest and nothing to declare. Ms. Patterson, Dr. Siegel, Dr. Martin, and Dr. Quick are employees, and stock and options holders at Castle Biosciences, Inc. Ms. Hunt is on the speakers bureau at Castle Biosciences, Inc.
References
Berger, A. C., Davidson, R. S., Poitras, J. K., Chabra, I., Hope, R., Brackeen, A.,…Miller, A. R. (2016). Clinical impact of a 31-gene expression profile test for cutaneous melanoma in 156 prospectively and consecutively tested patients. Current Medical Research and Opinion, 32(9), 1599–1604. https://doi.org/10.1080/03007995.2016.1192997
Bollard, S. M., Casalou, C., & Potter, S. M. (2021). Gene expression profiling in melanoma: A view from the clinic. Cancer Treatment and Research Communications, 29, 100447. https://doi.org/10.1016/j.ctarc.2021.100447
Bruinooge, S. S., Pickard, T. A., Vogel, W., Hanley, A., Schenkel, C., Garrett-Mayer, E.,…Williams, S. F. (2018). Understanding the role of advanced practice providers in oncology in the United States. Journal of Oncology Practice, 14(9), e518–e532. https://doi.org/10.1200/JOP.18.00181
Chandler, Y., Schechter, C. B., Jayasekera, J., Near, A., O’Neill, S. C., Isaacs, C.,…Mandelblatt, J. S. (2018). Cost effectiveness of gene expression profile testing in community practice. Journal of Clinical Oncology, 36(6), 554–562. https://doi.org/10.1200/JCO.2017.74.5034
Cohen, P. R., & Kurzrock, R. (2022). Dermatologic disease-directed targeted therapy (D3T2): The application of biomarker-based precision medicine for the personalized treatment of skin conditions-precision dermatology. Dermatology and Therapy, 12, 2249–2271.
Dillon, L. D., Gadzia, J. E., Davidson, R. S., McPhee, M., Covington, K. R., Cook, R. W.,…Fleming, M. D. (2018). Prospective, multicenter clinical impact evaluation of a 31-gene expression profile test for management of melanoma patients. SKIN: The Journal of Cutaneous Medicine, 2(2), 111–121. https://doi.org/10.25251/skin.2.2.3
Farberg, A. S., Glazer, A. M., White, R., & Rigel, D. S. (2017). Impact of a 31-gene expression profiling test for cutaneous melanoma on dermatologists’ clinical management decisions. Journal of Drugs in Dermatology, 16(5), 428–431. https://pubmed.ncbi.nlm.nih.gov/28628677/
Ferris, L. K., Farberg, A. S., Middlebrook, B., Johnson, C. E., Lassen, N., Oelschlager, K. M.,…Gerami, P. (2017). Identification of high-risk cutaneous melanoma tumors is improved when combining the online American Joint Committee on Cancer Individualized Melanoma Patient Outcome Prediction Tool with a 31-gene expression profile-based classification. Journal of the American Academy of Dermatology, 76(5), 818–825.e3. https://doi.org/10.1016/j.jaad.2016.11.051
Field, M. G., & Harbour, J. W. (2014). Recent developments in prognostic and predictive testing in uveal melanoma. Current Opinion in Ophthalmology, 25(3), 234–239. https://doi.org/10.1097/ICU.0000000000000051
Gerami, P., Cook, R. W., Wilkinson, J., Russell, M. C., Dhillon, N., Amaria, R. N.,…Stone, J. F. (2015a). Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clinical Cancer Research, 21(1), 175–183. https://doi.org/10.1158/1078-0432.CCR-13-3316
Gerami, P., Cook, R. W., Russell, M. C., Wilkinson, J., Amaria, R. N., Gonzalez, R.,…Lawson, D. (2015b). Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. Journal of the American Academy of Dermatology, 72(5), 780–5.e3. https://doi.org/10.1016/j.jaad.2015.01.009
Gershenwald, J. E., Scolyer, R. A., Hess, K. R., Thompson, J. F., Long, G. V., & Ross, M. I. (2017). AJCC cancer staging manual. In: 8th Edition AJCC Melanoma Staging System. Switzerland: Springer; 2017. p. 563–589.
Greenhaw, B. N., Zitelli, J. A., & Brodland, D. G. (2018). Estimation of prognosis in invasive cutaneous melanoma: An independent study of the accuracy of a gene expression profile test. Dermatologic Surgery, 44(12), 1494–1500. https://doi.org/10.1097/DSS.0000000000001588
Hijazo-Pechero, S., Alay, A., Marín, R., Vilariño, N., Muñoz-Pinedo, C., Villanueva, A.,…Solé, X. (2021). Gene expression profiling as a potential tool for precision oncology in non-small cell lung cancer. Cancers, 13(19), 4734. https://doi.org/10.3390/cancers13194734
Hsueh, E. C., DeBloom, J. R., Lee, J. H., Sussman, J. J., Covington, K. R., Caruso, H. G.,…McMasters, K. M. (2021). Long-term outcomes in a multicenter, prospective cohort evaluating the prognostic 31-gene expression profile for cutaneous melanoma. JCO Precision Oncology, 5(5), 589–601. https://doi.org/10.1200/PO.20.00119
Jarell, A., Gastman, B. R., Dillon, L. D., Hsueh, E. C., Podlipnik, S., Covington, K. R.,…Puig, S. (2022). Optimizing treatment approaches for patients with cutaneous melanoma by integrating clinical and pathologic features with the 31-gene expression profile test. Journal of the American Academy of Dermatology, 87(6), 1312–1320. https://doi.org/10.1016/j.jaad.2022.06.1202
Keller, J., Schwartz, T. L., Lizalek, J. M., Chang, E. S., Patel, A. D., Hurley, M. Y., & Hsueh, E. C. (2019). Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Medicine, 8(5), 2205–2212. https://doi.org/10.1002/cam4.2128
Lawson, D. H., Cook, R. W., Johnson, C., Russell, M. C., Amaria, R. N., Wilkinson, J.,…Lyle, S. (2015). Continued evaluation of a 31-gene expression profile test (GEP) for prediction of distant metastasis (DM) in cutaneous melanoma (CM). Journal of Clinical Oncology, 33(15_suppl), 9066. https://doi.org/10.1200/jco.2015.33.15_suppl.9066
Marson, J., Litchman, G., Svoboda, R., Glazer, A., Farberg, A., Winkelmann, R., & Rigel, D. S. (2021). Assessment of the 31-gene expression profile test by dermatologists: A cross-sectional survey from national dermatology conferences. SKIN: The Journal of Cutaneous Medicine, 5(2), 101–107. https://doi.org/10.25251/skin.5.2.4
Mirsky, R., Prado, G., Svoboda, R., Glazer, A., & Rigel, D. (2018). Management decisions made by physician assistants and nurse practitioners in cutaneous malignant melanoma patients: Impact of a 31-gene expression profile test. Journal of Drugs in Dermatology, 17(11), 1220–1223. https://pubmed.ncbi.nlm.nih.gov/30500144/
Morton, D. L., Thompson, J. F., Cochran, A. J., Mozzillo, N., Nieweg, O. E., Roses, D. F.,…MSLT Group (2014). Final trial report of sentinel-node biopsy versus nodal observation in melanoma. The New England Journal of Medicine, 370(7), 599–609. https://doi.org/10.1056/NEJMoa1310460
National Cancer Institute. (2023). Cancer Stat Facts: Melanoma of the skin. https://seer.cancer.gov/statfacts/html/melan.html
Podlipnik, S., Carrera, C., Boada, A., Richarz, N. A., López-Estebaranz, J. L., Pinedo-Moraleda, F.,…Puig, S. (2019). Early outcome of a 31-gene expression profile test in 86 AJCC stage IB-II melanoma patients. A prospective multicentre cohort study. Journal of the European Academy of Dermatology and Venereology, 33(5), 857–862. https://doi.org/10.1111/jdv.15454
Ross, A. C., Polansky, M. N., Parker, P. A., & Palmer, J. L. (2010). Understanding the role of physician assistants in oncology. Journal of Oncology Practice, 6(1), 26–30. https://doi.org/10.1200/JOP.091062
Schuitevoerder, D., Heath, M., Cook, R. W., Covington, K. R., Fortino, J., Leachman, S., & Vetto, J. T. (2018). Impact of gene expression profiling on decision-making in clinically node negative melanoma patients after surgical staging. Journal of Drugs in Dermatology, 17(2), 196–199.
Sheringham, J., King, A., Plackett, R., Khan, A., Cornes, M., & Kassianos, A. P. (2021). Physician associate/assistant contributions to cancer diagnosis in primary care: a rapid systematic review. BMC Health Services Research, 21(1), 644. https://doi.org/10.1186/s12913-021-06667-y
Sun, L., Wu, A., Bean, G. R., Hagemann, I. S., & Lin, C. Y. (2021). Molecular testing in breast cancer: Current status and future directions. The Journal of Molecular Diagnostics, 23(11), 1422–1432. https://doi.org/10.1016/j.jmoldx.2021.07.026
Ward, S., Scope, A., Rafia, R., Pandor, A., Harnan, S., Evans, P., & Wyld, L. (2013). Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: A systematic review and cost-effectiveness analysis. Health Technology Assessment, 17(44), 1–302. https://doi.org/10.3310/hta17440
Whitman, E. D., Koshenkov, V. P., Gastman, B. R., Lewis, D., Hsueh, E. C., Pak, H.,…Vetto, J. T. (2021). Integrating 31-gene expression profiling with clinicopathologic features to optimize cutaneous melanoma sentinel lymph node metastasis prediction. JCO Precision Oncology, 5, 1466–1479. https://doi.org/10.1200/PO.21.00162
Xin, L., Liu, Y. H., Martin, T. A., & Jiang, W. G. (2017). The era of multigene panels comes? The clinical utility of Oncotype DX and MammaPrint. World Journal of Oncology, 8(2), 34–40. https://doi.org/10.14740/wjon1019w