Research and Scholarship
Characterization and Management of Adverse Reactions in Patients With Unresectable Hepatocellular Carcinoma Treated With Lenvatinib
Anna Jones,(1) FNP-C, Paola Degregorio,(2) PA-C, Max W. Sung,(3) MD,
Zahra Ramji,(4) BSN, Min Ren,(5) PhD, and Ari D. Baron,(1) MD
From (1)Pacific Hematology Oncology Associates, San Francisco, California; (2)Mount Sinai Medical Center, New York, New York; (3)Tisch Cancer Institute at Mount Sinai, New York, New York; (4)Oncology Business Group, Eisai Inc., Nutley, New Jersey (Former Affiliation); (5)Clinical Research, Eisai Inc., Nutley, New Jersey
Authors’ disclosures of conflicts of interest are found at the end of this article.
Corresponding to: Anna Jones, FNP-C, Pacific Hematology Oncology Associates, 2100 Webster St. #225, San Francisco, CA 94115
E-mail: annaatkinsjones@gmail.com
J Adv Pract Oncol 2023;14(7):598–607 |
https://doi.org/10.6004/jadpro.2023.14.7.4 |
© 2023 BroadcastMed LLC

 ABSTRACT
ABSTRACT
Aims: Advanced practice providers (APPs) play a vital role in monitoring for and managing adverse reactions (ARs). As lenvatinib ARs can resemble cirrhosis (commonly presenting with hepatocellular carcinoma [HCC]), APP input is important for timely detection and management of ARs and to promote medication adherence. Design: The goal of this post-hoc analysis of the REFLECT trial was to characterize key ARs associated with lenvatinib, and to discuss management strategies. Methods: In REFLECT, patients with unresectable HCC were randomized to either daily lenvatinib (12 mg/day for patients who weighed ≥ 60 kg or 8 mg/day for those < 60 kg) or sorafenib 400 mg twice daily. Adverse events in the lenvatinib arm were grouped into ARs (hypertension, fatigue, palmar-plantar erythrodysesthesia syndrome, proteinuria, and decreased appetite) per the United States Prescribing Information (USPI) for lenvatinib. Results: Key ARs in the lenvatinib arm (n = 476) generally occurred within months of starting lenvatinib. Some cases of proteinuria, decreased appetite, and diarrhea were first reported at about 2 years of treatment. Conclusions: The onset of key ARs associated with lenvatinib treatment can be predicted and generally be managed (per the lenvatinib USPI and REFLECT) by withholding lenvatinib and resuming it at a reduced dose after the severity decreases. However, lenvatinib should generally be discontinued if the AR is life-threatening.
ARTICLE
In 2023, liver cancer accounted for 2.1% and 4.8% of estimated new cancer cases and cancer deaths in the United States, respectively (National Cancer Institute, 2023). Globally, in 2020, liver cancer was reported as 4.7% of new cancer cases but 8.3% of cancer deaths (Sung et al., 2021). Hepatocellular carcinoma (HCC) specifically accounts for up to 85% of liver cancer cases and has a 5-year survival rate below 30% (Aly et al., 2020).
Lenvatinib (Lenvima) is an oral multikinase inhibitor approved in over 70 countries as a first-line therapy for patients with advanced or unresectable HCC (uHCC; Eisai Co., Ltd., 2021) based on results from the phase III multicenter REFLECT trial (Eisai GmbH, 2023; Eisai Inc., 2023; Kudo et al., 2018). In REFLECT, the median overall survival with lenvatinib was 13.6 months (95% confidence interval [CI] = 12.1–14.9) compared with 12.3 months (95% CI = 10.4–13.9) with sorafenib; lenvatinib met the primary endpoint of noninferiority compared with sorafenib (hazard ratio, 0.92; 95% CI = 0.79–1.06; Kudo et al., 2018). Lenvatinib showed a statistically significant improvement vs. sorafenib for efficacy endpoints of progression-free survival, time to progression, and objective response rate (all p < .0001; Kudo et al., 2018).
Among patients treated with lenvatinib (n = 476) in REFLECT, 99% experienced treatment-emergent adverse events (TEAEs) of any severity grade, and 75% experienced TEAEs of grade ≥ 3 (Kudo et al., 2018). Treatment-related TEAEs led to dose interruptions (40%), reductions (37%), and withdrawals (9%) in the lenvatinib-treated patients.
Adverse event (AE) recognition and management are key to lenvatinib dose modification (interruption or reduction) and discontinuation. Maintaining lenvatinib doses should optimize treatment benefit for patients. However, HCC often develops against a background of cirrhosis with associated systemic manifestations, including fatigue, decreased appetite, nausea, and weight loss (Sun & Sarna, 2008), which can pose challenges in the diagnosis and management of AEs. Previous manuscripts have summarized adverse events and reactions associated with lenvatinib for the treatment of radioiodine-refractory differentiated thyroid cancer (Cabanillas & Takahashi, 2019) and endometrial carcinoma (Makker et al., 2021). Thus, we conducted a post-hoc analysis to characterize key AEs associated with lenvatinib for the treatment of uHCC. Adverse events were grouped as adverse reactions (ARs) based on the US Food and Drug Administration’s definition. Other goals were to describe the respective management strategies for key ARs in patients with uHCC based on the United States Prescribing Information (USPI) and the REFLECT trial to highlight the critical role of advanced practice providers (APPs) as a primary contact for patient education on ARs, and to provide APP perspectives on the management of these challenging ARs.
Methods
Study Design and Patients
The study design of REFLECT and patient eligibility criteria have been described previously (Kudo et al., 2018) and are summarized in Figure 1. Briefly, patients with uHCC with ≥ 1 measurable target lesion and no prior systemic treatment were randomized 1:1 to receive lenvatinib or sorafenib, administered in 28-day cycles. Lenvatinib was dosed based on patient body weight: 12 mg/day for patients weighing ≥ 60 kg or 8 mg/day for those weighing < 60 kg. The sorafenib dosage was 400 mg twice daily. Patients provided informed consent prior to enrollment (Kudo et al., 2018).
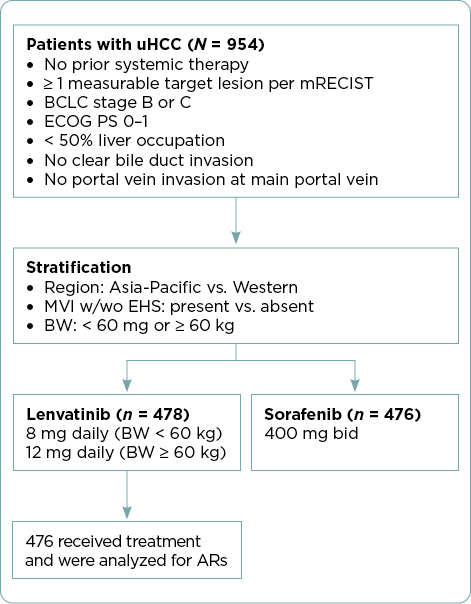
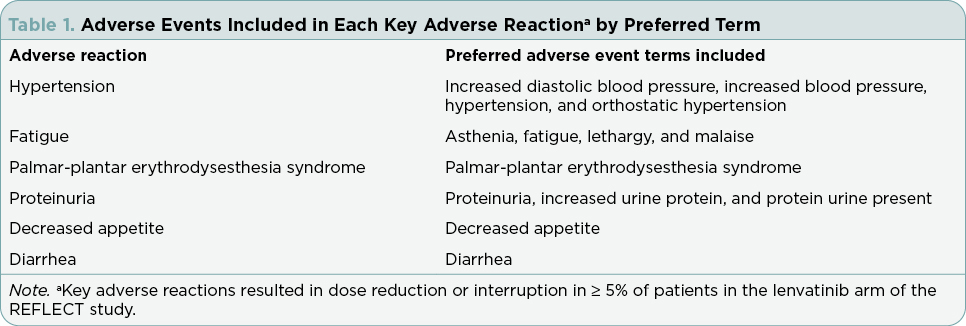
All lenvatinib-treated patients were included in this post-hoc analysis of REFLECT. Key ARs leading to dose reductions or interruptions of lenvatinib in ≥ 5% of patients are shown in Table 1 and applied in accordance with lenvatinib’s USPI (Eisai Inc., 2023). Recommendations for the management of these key ARs and strategies educating patients about key ARs are described from the perspective of APPs.
Results
Patient Demographics
Select patient demographics and baseline characteristics of patients in the lenvatinib arm of REFLECT have been published (Kudo et al., 2018). The median age of patients in the lenvatinib arm was 63 years, and 85% were men. Most patients in this arm weighed ≥ 60 kg (68%), had an Eastern Cooperative Oncology Group performance status of 0 (64%), and Barcelona Clinic Liver Cancer stage C disease (advanced; 78%). Most patients (53%) had chronic liver disease attributed to hepatitis B; other causes included hepatitis C (19%), alcohol (8%), or another cause (8%). Underlying cirrhosis (per masked independent imaging review) was present in the majority (74%) of patients in the lenvatinib arm. Of the 478 patients randomly assigned to lenvatinib, 476 received treatment and were included in the safety analysis. The median duration of lenvatinib treatment was 5.7 months (interquartile range, 2.9–11.1; Kudo et al., 2018).
Types, Frequency, and Characterization of ARs
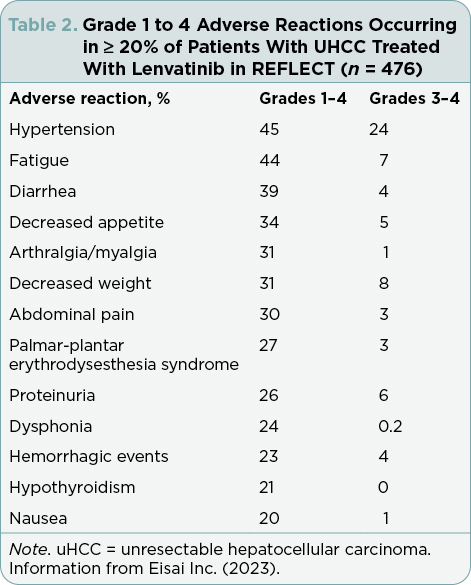
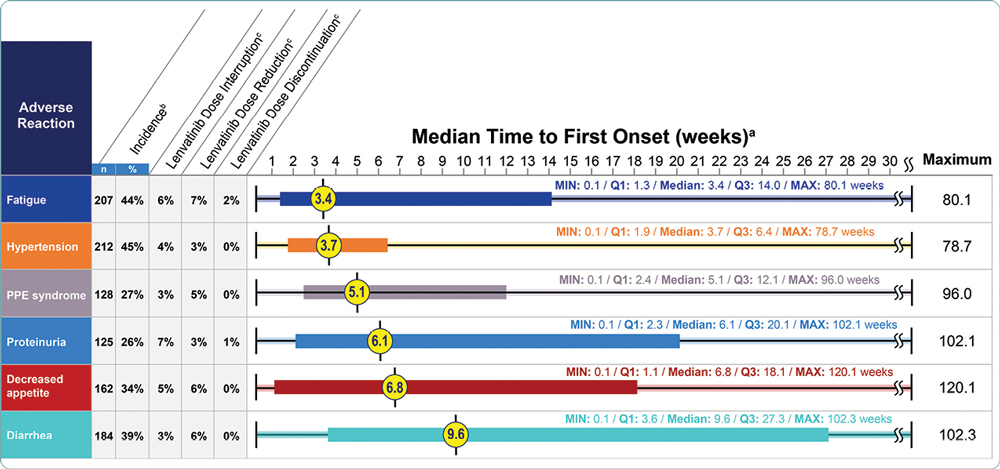
Grade 1 to 4 ARs that occurred in ≥ 20% of patients treated with lenvatinib in REFLECT are shown in Table 2. The most common (≥ 5%) ARs resulting in dose reductions or interruptions of lenvatinib were analyzed, including fatigue, hypertension, palmar-plantar erythrodysesthesia (PPE) syndrome, proteinuria, decreased appetite, and diarrhea. Median times to first onset of key ARs occurred within the first 10 weeks of treatment (Figure 2). Details for each of the key ARs are provided.
Strategies to Manage Key Treatment-Related Adverse Reactions
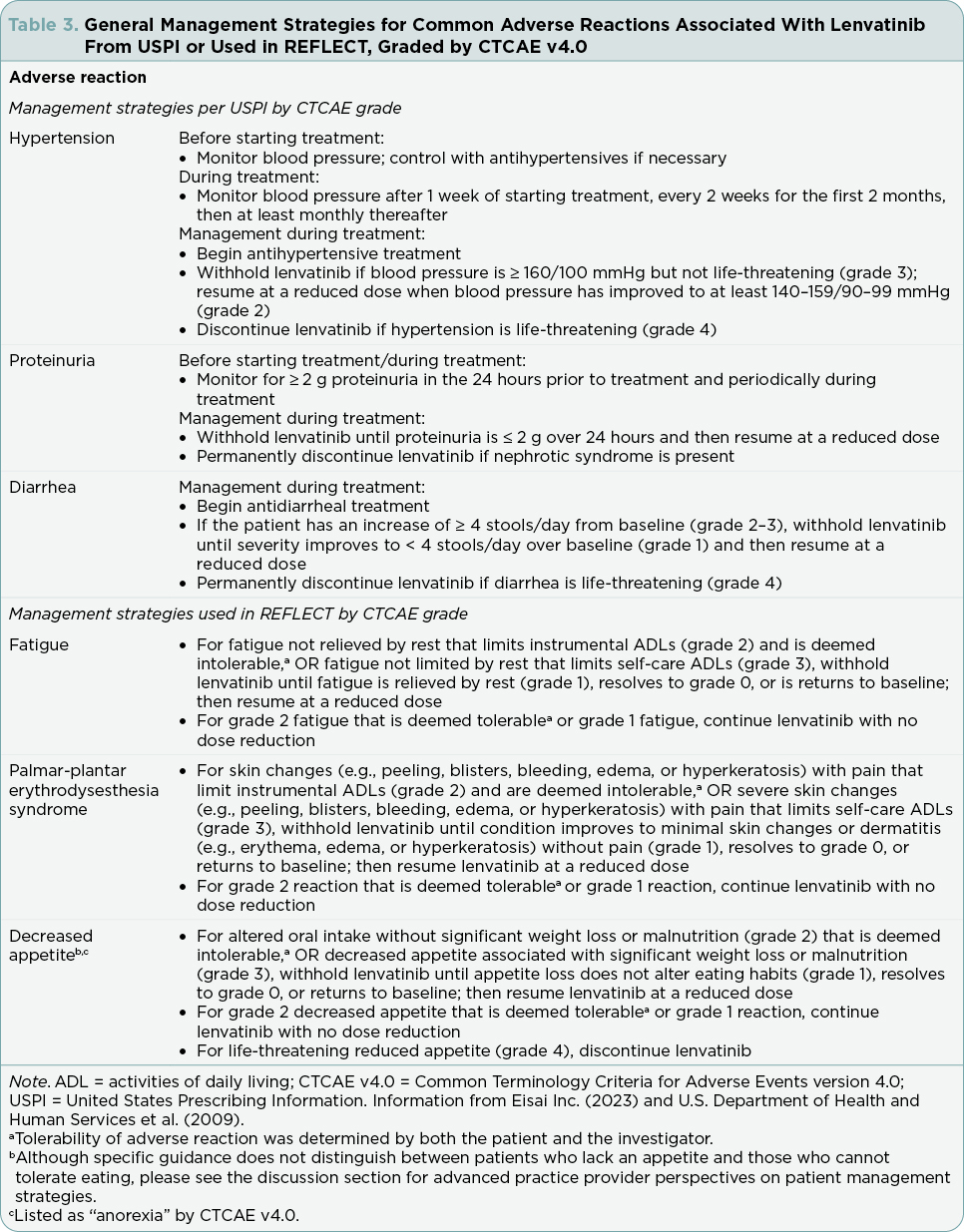
Dose modification strategies (reduction or interruption) or withdrawal should be used to manage lenvatinib ARs; these strategies from the lenvatinib USPI (Eisai Inc., 2023) are summarized in the following section. For patients weighing ≥ 60 kg, the lenvatinib dose should be reduced to 8 mg/day (first decrease), 4 mg/day (second), and 4 mg every other day (third). For patients weighing < 60 kg, the dose should be lowered to 4 mg/day (first decrease) and then 4 mg every other day (second). Lenvatinib should be discontinued if additional dose reductions are required. General advice for the management of ARs is summarized in Table 3; additional details can be found in the lenvatinib USPI (Eisai Inc., 2023). Health-care providers (HCPs) should refer to their local prescribing information for additional information related to lenvatinib-related warnings and reactions, as well as specific management strategies.
Hypertension
Hypertension was one of the most common grade 1 to 4 ARs in REFLECT, occurring in 45% of patients receiving lenvatinib (Table 2). Overall, hypertension led to lenvatinib dose interruptions in 4% of patients and to dose reductions in 3% (Figure 2). The median time to first onset of hypertension was 3.7 weeks. Hypertension may be associated with the mechanism of action of tyrosine kinase inhibitors (TKIs; Kandula & Agarwal, 2011), and patients were required to have adequately controlled hypertension (i.e., blood pressure ≤ 150/90 mmHg), with or without antihypertensive medications, at study entry (Kudo et al., 2018).
Fatigue
In REFLECT, fatigue was the second most common grade 1 to 4 AR, occurring in 44% of patients receiving lenvatinib (Table 2). Of the key ARs, fatigue had the shortest median time to onset (3.4 weeks) and most commonly led to dose reductions (7%) and discontinuations (2%; Figure 2).
PPE Syndrome
Grade 1 to 4 PPE syndrome was reported in 27% of patients receiving lenvatinib in REFLECT (Table 2). The median time to first onset of PPE syndrome was 5.1 weeks, and most cases occurred within the first 3 months (Figure 2). Overall, PPE syndrome led to lenvatinib dose interruptions in 3% of patients and to dose reductions in 5%.
Proteinuria
In REFLECT, 26% of patients receiving lenvatinib had grade 1 to 4 proteinuria (Table 2) and the median time to first onset was 6.1 weeks (Figure 2). Proteinuria led to lenvatinib dose interruptions in 7% of patients and discontinuations in 1%.
Decreased Appetite
In REFLECT, 34% of patients receiving lenvatinib had a grade 1 to 4 AR of decreased appetite (Table 2). Decreased appetite had the largest range of time to first onset of the key ARs, from 0.1 to 120.1 weeks, and a median time to first onset of 6.8 weeks (Figure 2).
Diarrhea
In REFLECT, 39% of patients treated with lenvatinib had a grade 1 to 4 AR of diarrhea (Table 2). Diarrhea had the longest median time to first onset (9.6 weeks; Figure 2).
Other Notable Conditions
Hepatic encephalopathy occurred in 8% of patients who received lenvatinib in REFLECT and was grade 3 to 4 in 4%. Hepatic encephalopathy led to lenvatinib discontinuation in 2% of patients.
Discussion
APP Perspectives on Patient Management: At a Glance
As many patients with HCC have underlying cirrhosis—which has overlapping manifestations with lenvatinib ARs—APPs (or other authorized HCPs) should evaluate patients for preexisting conditions, such as fatigue, decreased appetite, depression, insomnia, thyroid irregularities, and weight loss prior to initiating lenvatinib. Hypertension and proteinuria should also be screened for and managed before therapy initiation. The onset of ARs can generally be expected early into treatment, which can help differentiate ARs from manifestations of cirrhosis. It is essential that APPs/authorized HCPs frequently follow up with patients to offer support and to ensure that any newly developed ARs do not go unreported. During follow-up, HCPs should advise and encourage patients to adhere to the medication for the required duration of treatment. Generally, ARs can be managed with standard therapy and/or lenvatinib dose modifications; however, patients should be referred to a hepatologist, primary care physician, cardiologist, nephrologist, palliative care team, endocrinologist, psychiatrist, or dietitian when needed as part of a multidisciplinary approach. Considering the involvement of cirrhosis in uHCC, APP/HCPs should also be aware of liver toxicities known to occur with lenvatinib, such as hepatic encephalopathy (Eisai Inc., 2023), and should refer to their local PI for management strategies.
APP Perspectives on the Management of Hypertension and Proteinuria
Before starting lenvatinib, APPs/HCPs should ask patients about conditions such as preexisting hypertension and ensure that patients’ blood pressure is controlled. As lenvatinib-induced hypertension can occur early on, blood pressure should be checked 1 week after treatment commences, every 2 weeks for about 2 months, and then monthly for the remainder of treatment (Eisai Inc., 2023). Particularly, patients with preexisting hypertension or other cardiovascular issues may need to monitor their blood pressure daily at least for the first 3 to 4 weeks of lenvatinib therapy. This can be done in the clinic or at home by patients, if properly demonstrated. It is best to provide patients with a logbook to record their blood pressure readings. Patients and their caregivers should be educated on parameters for hypertension that warrant contacting their HCP for further management, i.e., a blood pressure reading of > 140/90 mmHg for ≥ 3 consecutive readings. Other causes of elevated blood pressure, such as concurrent pain, anxiety, recent caffeine intake, or physical exertion prior to blood pressure assessment, should also be considered.
Proteinuria may be associated with hypertension (Kandula & Agarwal, 2011); it should be screened for before and during treatment (Eisai Inc., 2023). Proteinuria is typically detected through a 24-hour urine collection; while a urine dipstick can be used in lieu of a 24-hour urine collection, the latter should be considered if dipstick results are 2+ or greater. Lenvatinib should be withheld when ≥ 2 grams of protein are collected over 24 hours and discontinued in the presence of nephrotic syndrome (Eisai Inc., 2023).
Angiotensin-converting enzyme inhibitors may be considered, as they are generally recommended among first-line pharmacotherapies for hypertension (Unger et al., 2020; Whelton et al., 2018), and are recommended for treatment of blood pressure occurring with chronic kidney disease and albuminuria (Unger et al., 2020; Whelton et al., 2018; Williams et al., 2018). Caution is advised if providing diuretics for blood pressure control, especially if a patient presents with concurrent diarrhea. Patients with preexisting hypertension may require additional treatment and should be referred to a cardiologist if their blood pressure is difficult to control despite optimizing antihypertensive treatments. Patients should be educated about when to seek immediate medical attention for potential signs and symptoms suggestive of hypertensive urgency and emergency, including severe headache, visual changes, and intractable nausea or vomiting.
APP Perspectives on the Management of Fatigue and Decreased Appetite
The baseline level of fatigue (from factors such as the illness itself, depression, insomnia, or hypothyroidism) should be assessed before lenvatinib initiation to determine if it worsens during treatment. Fatigue can be evaluated and monitored subjectively by asking questions, such as how it is affecting the patient’s quality of life and their activities of daily living (ADLs), and objectively, by using instrumental assessments or questionnaires such as the Brief Fatigue Inventory (The University of Texas MD Anderson Cancer Center, 1999). Treatable causes of preexisting fatigue should be addressed prior to and during lenvatinib treatment. Advanced practice providers/authorized HCPs should note that sleep disruption (such as from nocturia), thyroid disorders, and mood disorders may contribute to fatigue, and thus referral to a specialist may be necessary.
If fatigue is considered related to lenvatinib, treatment modification strategies depend on its severity. Common Terminology Criteria for Adverse Events (CTCAE) guidelines should be considered to grade fatigue severity. Patients should be advised to incorporate rest periods into their daily schedule. Mild to moderate exercise is recommended, if tolerated. Patients should also be counseled that fatigue is a common side effect of lenvatinib.
Decreased appetite is a lenvatinib AR that may also contribute to fatigue; thus, referral to a dietitian may be warranted. Advanced practice providers/authorized HCPs should determine if decreased appetite stems from a general lack of appetite or an intolerance to specific foods. Dietary strategies are particularly helpful; specifically, patients may be able to better tolerate small, frequent meals of high-calorie foods. Light weight-bearing exercises may also help to manage fatigue and decreased appetite.
APP Perspectives on the Management of PPE Syndrome
To prevent PPE syndrome, patients should be counseled to remove calluses prior to initiating lenvatinib but to stop callus removal once treatment has begun. When starting treatment, patients should be advised to apply moisturizer to their palms and soles. They should also be counseled to avoid contact with hot water; rough or tight-fitting clothing, footwear, and jewelry; harsh chemicals (including alcohol-based products); and prolonged sunlight (BC Cancer, 2019; Rimassa et al., 2019), as all can intensify heat and skin irritation. Patients should be taught to conduct daily self-assessments of their skin and to understand the early signs of PPE syndrome, including tingling, numbness, and areas of dry, furrowed skin with any changes in pigmentation. Advanced practice providers/authorized HCPs should reinforce when patients should seek immediate medical attention, such as the occurrence of fever, redness, or discharge/odor from any skin breaks or open areas, inability to perform ADLs; or when pain is not controlled (BC Cancer, 2019).
Authorized HCPs, including APPs, should evaluate lenvatinib-treated patients for PPE syndrome on a similar schedule to blood pressure follow-up visits (i.e., 1 week after starting lenvatinib, every 2 weeks for 2 months, and then at least monthly), or when PPE syndrome worsens. In addition to in-person visits, evaluation can be performed through telehealth video calls or phone calls where the patient sends photos of affected skin separately. If symptoms appear, patients should be counseled to use keratolytics containing urea cream and salicylic acid to remove excess skin (however, once taking lenvatinib, no mechanical debridement should be used; BC Cancer, 2019; Rimassa et al., 2019). Patients may also need to apply a topical steroid cream twice daily.
Grade 2 severity PPE syndrome is considered urgent, requiring intervention within 24 hours, and warranting complete assessment of the lesions as well as a vital sign check to ensure that infection is not present. Patients should be evaluated for pain, and APPs should manage pain symptoms appropriately, such as with topical lidocaine (unless blisters or bleeding are present) and, if refractory, with systemic analgesics. For management of PPE syndrome, APPs should review CTCAE guidelines for grading.
APP Perspectives on the Management of Diarrhea
Some patients have preexisting diarrhea from past conditions or ongoing medications; it is helpful to know the baseline level of diarrhea and a description of its symptoms so that preexisting diarrhea is managed prior to lenvatinib initiation. Concomitant medications associated with diarrhea should be monitored. Dose modification of these medications may be warranted if patients develop diarrhea during lenvatinib therapy, although other reasons for diarrhea (lactose intolerance, gluten sensitivity, celiac sprue, infectious diarrhea, and inflammatory colitis) should be considered. Patients should be counseled to use antidiarrheals at first onset of loose stool. They should also be advised to contact the clinic at the onset of loose stool so that they can be followed closely to ensure that diarrhea becomes well controlled. Patients should be advised to note the difference between loose but formed stool and watery diarrhea, as the latter is a more serious AR of lenvatinib. If patients develop a predictable pattern of diarrhea, they should be counseled to use antidiarrheals preemptively, about 30 minutes prior to taking lenvatinib (Rimassa et al., 2019). Patients should be counseled on the “bananas, rice, applesauce, and toast” (BRAT) diet to solidify stools and to ensure sufficient hydration to replenish electrolytes lost from diarrhea. Patients with chronic diarrhea should be referred to a dietitian to help manage their nutrition. Patients who experience diarrhea should avoid caffeine and dairy. Those with acute or worsening diarrhea should be evaluated for infection, and appropriate diagnostic tools and treatments for infection should be implemented.
For patients with acute or severe diarrhea, fluid and electrolyte status should be assessed by obtaining vital signs, complete metabolic panel, and magnesium levels, and abnormalities should be managed as necessary. Dose modifications of lenvatinib depend on the severity of diarrhea. Lenvatinib USPI (Eisai Inc., 2023) recommendations should be followed. If symptoms are severe, lenvatinib should be withheld until symptoms return to baseline. APPs should reinforce when to seek immediate medical attention, including in the presence of fever, abdominal pain, dizziness, large loose stools (≥ 4/day), blood or pus in stool, or nausea/vomiting.
Patient-Education Considerations Based on Nursing Experience
As part of a multidisciplinary team, APPs are often one of the primary points of contact for patient education, including expectations for lenvatinib treatment, means of administration, typical ARs and their expected timing, potential interactions of lenvatinib with concomitant medications or with grapefruit, and AR management. Patients often have concerns about lenvatinib and its potential ARs, so it is crucial to reassure them that they will receive the optimal tolerated dose. This usually enables patients to stay on treatment longer and derive maximum therapeutic benefit. As the amount of information patients receive initially can be overwhelming and difficult to remember, it is important to provide them with resources such as the Oral Chemotherapy Education sheet (Hematology/Oncology Pharmacy Association, 2021), which has useful information about common side effects and explains what to do if patients experience them. At the end of the patient education visit, the patient and caregiver should be able to repeat back information reviewed to ensure that they understand and remember the important points. Clinics may want to consider developing their own patient education materials on management of key ARs, such as diarrhea, fatigue, and hypertension.
Advanced practice providers should review the common ARs with patients and discuss how they can be managed at home. It is also essential to inform patients when it is important to call/visit the clinic for symptom management, as well as when to call emergency services. Telemedicine and telephone follow-ups are also important tools for reviewing and reinforcing drug-related information, including side effects and their management. Telemedicine can also be used to assess PPE syndrome, skin rashes, and blood pressure in a timely fashion, without the patient having to visit the clinic.
Implications for Practice
Lenvatinib appears to be an effective first-line treatment for patients with uHCC. However, almost all REFLECT participants experienced TEAEs, and 75% experienced TEAEs of grade ≥ 3 severity (Kudo et al., 2018); many of which can result in premature discontinuation if poorly managed. Additionally, HCC often develops against a background of cirrhosis, which has associated systemic manifestations that overlap with lenvatinib’s side-effect profile. Although this potential dual origin can complicate patient management, the early onset of ARs as shown in our analysis can help to differentiate between treatment- and disease-related AEs. As APPs are one of the primary contacts for patients and caregivers, they play a pivotal role in appropriately identifying potentially concerning AEs. These contacts, combined with frequent routine follow-up, help to ensure that patients do not discontinue lenvatinib prematurely and, therefore, help to optimize treatment outcomes.
Disclosure
This study was sponsored by Eisai Inc., Nutley, NJ, USA, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Medical writing support was provided by Michael Venditto, PharmD, of Oxford PharmaGenesis Inc., Newtown, PA, USA, and was funded by Eisai Inc., Nutley, NJ, USA, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Ms. Jones and Ms. Degregorio have no conflicts of interest to disclose. Dr. Sung has served a consulting or advisory role for Bayer, Eisai, Exelixis, and Genentech. Ms. Ramji and Dr. Ren are employees of Eisai Inc. Dr. Baron has served on the speakers bureau for Lilly, Amgen, Eisai, Johnson & Johnson, AbbVie, Merck, and Bristol Myers Squibb.
References
Aly, A., Ronnebaum, S., Patel, D., Doleh, Y., & Benavente, F. (2020). Epidemiologic, humanistic and economic burden of hepatocellular carcinoma in the USA: A systematic literature review. Hepatic Oncology, 7(3), HEP27. https://doi.org/10.2217/hep-2020-0024
BC Cancer. (2019). Symptom management guidelines: Palmar-plantar erythrodysesthesia (PPE). http://www.bccancer.bc.ca/nursing-site/Documents/14.%20Palmar%20Plantar%20Erythrodysesthesia.pdf
Cabanillas, M. E., & Takahashi, S. (2019). Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Seminars in Oncology, 46(1), 57–64. https://doi.org/10.1053/j.seminoncol.2018.11.004
Eisai Co., Ltd. (2021). New data on Lenvima® (lenvatinib) plus Keytruda® (pembrolizumab) versus sunitinib in first-line treatment for patients with advanced renal cell carcinoma from pivotal phase 3 CLEAR/KEYNOTE-581 trial presented at 2021 ASCO Annual Meeting [press release]. https://www.eisai.com/news/2021/news202140.html
Eisai GmbH. (2023). Lenvima 4 mg and 10 mg hard capsules [summary of product characteristics]. Frankfurt am Main, Germany: Eisai GmbH.
Eisai Inc. (2023). Lenvima (lenvatinib) package insert. https://www.lenvima.com/-/media/Project/EIsai/Lenvima/PDF/prescribing-information.pdf?hash=8e823de7-4207-411d-8b00-48ac4ea27356&v=2022-11
Hematology/Oncology Pharmacy Association. (2021). Oral chemotherapy education: Lenvatinib. https://www.oralchemoedsheets.com/sheets/Lenvatinib_Patient_Education.pdf
Kandula, P., & Agarwal, R. (2011). Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney International, 80(12), 1271–1277. https://doi.org/10.1038/ki.2011.288
Kudo, M., Finn, R. S., Qin, S., Han, K. H., Ikeda, K., Piscaglia, F.,...Cheng, A. L. (2018). Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet, 391(10126), 1163–1173. https://doi.org/10.1016/S0140-6736(18)30207-1
Makker, V., Taylor, M. H., Oaknin, A., Casado Herraez, A., Orlowski, R., Dutta, L.,...O’Malley, D. M. (2021). Characterization and management of adverse reactions in patients with advanced endometrial carcinoma treated with lenvatinib plus pembrolizumab. Oncologist, 26(9), e1599–e1608. https://doi.org/10.1002/onco.13883
National Cancer Institute. (2023). Cancer stat facts: Liver and intrahepatic bile duct cancer. Surveillance, Epidemiology, and End Results Program (SEER). https://seer.cancer.gov/statfacts/html/livibd.html
Rimassa, L., Danesi, R., Pressiani, T., & Merle, P. (2019). Management of adverse events associated with tyrosine kinase inhibitors: Improving outcomes for patients with hepatocellular carcinoma. Cancer Treatment Reviews, 77, 20–28. https://doi.org/10.1016/j.ctrv.2019.05.004
Sun, V. C., & Sarna, L. (2008). Symptom management in hepatocellular carcinoma. Clinical Journal of Oncology Nursing, 12(5), 759–766. https://doi.org/10.1188/08.CJON.759-766
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
The University of Texas MD Anderson Cancer Center. (1999). Brief Fatigue Inventory. https://www.ons.org/sites/default/files/2017-06/BriefFatigueInventory_English_1.pdf
U.S. Department of Health and Human Services, National Institutes of Health, & National Cancer Institute. (2010). Common terminology criteria for adverse events (CTCAE). Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
Unger, T., Borghi, C., Charchar, F., Khan, N. A., Poulter, N. R., Prabhakaran, D.,...Schutte, A. E. (2020). 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension, 75(6), 1334–1357. https://doi.org/10.1161/HYPERTENSIONAHA.120.15026
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Jr., Collins, K. J., Dennison Himmelfarb, C.,...Wright, J. T., Jr. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 71(6), e13–e115. https://doi.org/10.1161/HYP.0000000000000065
Williams, B., Mancia, G., Spiering, W., Agabiti Rosei, E., Azizi, M., Burnier, M.,...ESC Scientific Document Group. (2018). 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal, 39(33), 3021–3104. https://doi.org/10.1093/eurheartj/ehy339