Research and Scholarship
Capecitabine for Salvage Treatment of Patients With Heavily Pretreated Human Papillomavirus–Associated Oropharynx Cancer With Distant Metastases
Anna C. Cooper, PA-C, Casey A. Fazer, PA-C, Ashish V. Chintakuntlawar, MBBS, PhD, Harry E. Fuentes Bayne, MD, MS, Patrick W. McGarrah, MD, and Katharine A. R. Price, MD
From Division of Medical Oncology, Mayo Clinic, Rochester, Minnesota
Authors’ disclosures of conflicts of interest are found at the end of this article.
Correspondence to: Katharine A. R. Price, MD, Mayo Clinic, 200 First Street SW, Rochester, MN, 55902. Email: price.katharine@mayo.edu
J Adv Pract Oncol 2023;14(7):571–575 |
https://doi.org/10.6004/jadpro.2023.14.7.2 |
© 2023 BroadcastMed LLC

 ABSTRACT
ABSTRACT
Background: Patients with metastatic human papillomavirus–associated oropharyngeal cancer (HPV-OPC) have a median overall survival exceeding 2 years and are often candidates for multiple lines of palliative therapy. With the approval of immunotherapy as first-line treatment, salvage therapeutic options are limited. We describe our experience using capecitabine as salvage therapy for patients with recurrent or metastatic (R/M) HPV-OPC. Methods: We performed a retrospective study of patients with R/M HPV-OPC with distant metastatic disease. Eligible patients were identified from a medical oncology clinical database. Demographic and clinical data were abstracted from the medical record. Survival probabilities were estimated with the Kaplan-Meier method. Results: 10 patients were identified. Sites of metastatic disease included lung, liver, mediastinal lymph nodes, bone, abdominal lymph nodes, and soft tissue. Most patients received capecitabine as fourth-line treatment. The median duration from start of capecitabine therapy until death was 10.5 months. Best treatment response was as follows: partial responses (PR) were seen in 4 of 10 (40%), stable disease (SD) in 3 of 10 (30%), and progressive disease (PD) in 2 of 10 (20%). The clinical benefit rate (CR + PR + SD) was 70%. Reasons for discontinuation included disease progression (n = 8) and side effects (n = 2). One patient notably had prolonged benefit from capecitabine and continued to be on treatment for 34 months total. Conclusions: Capecitabine is a potential salvage treatment for heavily pretreated patients with R/M HPV-OPC, with some patients experiencing prolonged response. Clinical or molecular predictors of response would be helpful to identify patients likely to benefit; a larger prospective study would be useful to confirm efficacy in this patient population.
ARTICLE
Head and neck squamous cell carcinoma (HNSCC) accounts for 4% of overall malignancies, with 66,920 patients diagnosed annually and 15,400 dying from their disease (Siegel et al., 2023). Despite a decrease in tobacco use, and the resultant decline in HNSCC overall, oropharyngeal cancer (OPC) has been increasing for the past 20 years due to the increase in human papillomavirus (HPV) infections in the oropharyngeal region (Van Dyne et al., 2018). Human papillomavirus causes about 70% of oropharyngeal cancers in the United States (Centers for Disease Control and Prevention [CDC], 2023). Transmission occurs through sexual and oral-genital contact (CDC, 2023). Human papillomavirus prevalence is three-fold more common in men compared with women, in part from higher numbers of lifetime sexual partners and stronger association with sexual behavior among men (Wierzbicka et al., 2021). Most HPV infections are cleared by the body within the first 2 years, but not all, and at least 15 of 100 viral types are highly oncogenic (You et al., 2019). Association is mainly with HPV 16, which accounts for over 90% of cases (Wierzbicka et al., 2021).
Although human papillomavirus–associated oropharyngeal cancers (HPV-OPC) generally have a more favorable prognosis than non-HPV–positive cancers, metastatic disease still occurs. The median time to development of metastases is significantly longer for HPV-positive tumors compared with HPV-negative tumors: 16.4 vs. 7.2 months, respectively (Sacks et al., 2020). In an analysis by Brkic and colleagues (2021), of 211 metastatic HPV-OPC patients, median overall survival (OS) of the total group was 13.5 months. The standard-of-care first-line regimen for patients with R/M HNSCC is fluorouracil (5-FU), along with platinum-based chemotherapy and pembrolizumab (Keytruda), or pembrolizumab alone based on the results of the KEYNOTE-048 study (Burtness et al., 2019).
Considering that HPV-OPC patients are relatively younger with fewer medical comorbidities and the incorporation of immunotherapy into front-line treatment, there is a relative dearth of salvage options. Even in the face of metastatic disease, patients with HPV-OPC can live for years and potentially be eligible for multiple lines of salvage systemic therapy. Currently, there is no standard of care in the second line or beyond for patients with R/M HPV-OPC.
According to the National Comprehensive Cancer Network (NCCN) Guidelines, single-agent capecitabine is a subsequent line of treatment in the metastatic setting, after first-line chemoimmunotherapy (NCCN Guidelines, 2022). Capecitabine is a prodrug of 5-FU, a pyrimidine antagonist, and is absorbed through the intestine. 5-FU has limitations, as the mechanism of the drug is limited by its rapid degradation in the body by the enzyme dihydropyrimidine dehydrogenase. This enzyme displays considerable variation from one to subject another, creating unpredictable bioavailability. The final activation stage of capecitabine requires thymidine phosphorylase, which is present in tumor cells in higher amounts compared with normal cells, and results in a much greater concentration of 5-FU in tumor cells (Wisniewska-Jarosinska et al., 2011). Capecitabine is an oral pill, which is a highly convenient option for patients and can be taken orally or through a feeding tube.
There are no published studies describing the utility of capecitabine specifically in patients with R/M HPV-OPC. We report on our experience using capecitabine as salvage therapy in heavily pretreated patients with R/M HPV-OPC.
Methods
This retrospective study was reviewed and approved by the Mayo Clinic Institutional Review Board (IRB). According to Minnesota state statutes, all living patients provided consent to review their medical records. Patients with R/M HPV-OPC who were treated and followed at Mayo Clinic between 2016 and 2020 were identified using a medical oncology clinical database. Demographic, pathologic, and clinical data were abstracted from the medical record, which included age at diagnosis, gender, tobacco history, date of diagnosis, p16 and HPV status, site of primary tumor, initial cancer treatment, date of diagnosis of metastatic disease, site(s) of metastatic disease, previous systemic therapies in the R/M setting, date of initiation of capecitabine, side effects of capecitabine, and reason and date for discontinuation of capecitabine treatment. Treatment-related variables included previous lines of systemic therapy, capecitabine dosing, and treatment schedule. Response to therapy was determined per investigator assessment using radiologic images.
Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 was not used due to poor resolution of the CT portion of previous PET-CT scans and the retrospective nature of the study. Overall response rate (ORR) was defined as patients with complete response (CR) and partial response (PR). Clinical benefit rate (CBR) was defined as patients with CR, PR, and stable disease (SD). Descriptive statistics were used to summarize the distribution of baseline parameters. Survival probabilities were calculated using the Kaplan-Meier method. Continuous variables were expressed by the median and interquartile range as appropriate. Overall survival (OS) was estimated from the date of documented R/M disease to the date of death or last follow-up. Survival on capecitabine was calculated from the initiation of therapy to the date of death or last follow-up. Patients were censored at their last follow-up or contact date if they were lost to follow-up. For all statistical analysis, we used BlueSky Statistics, version 7.4v (BlueSky Statistics LLC, Chicago, IL).
Results
The study population included 10 patients who received capecitabine as salvage therapy for R/M HPV-OPC. Patient characteristics are summarized in Table 1. The typical dose began at 1,250 mg/m2 twice daily for 14 days, followed by a 1-week rest period every 21 days. All patients were male. The median age at diagnosis was 63.5 years (range 57–76). The sites of metastatic disease were lung (n = 9), liver (n = 7), mediastinal lymph nodes (n = 5), bone (n = 4), abdominal lymph nodes (n = 2), and soft tissue (n = 1). The median age at follow-up was 68 years (range 59–82). The median time from diagnosis of metastatic disease to the start of capecitabine therapy was 20.3 months (range 12–32 months). The median fourth line of therapy for patients was capecitabine. Prior lines of therapy included platinum, taxanes, immunotherapy, anti–epidermal growth factor receptor (EGFR) therapy, clinical trials, and antimetabolites.
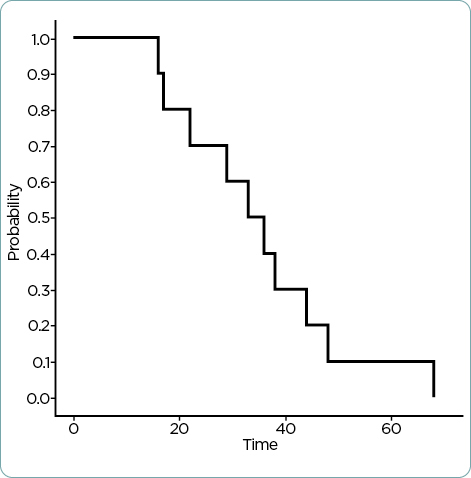
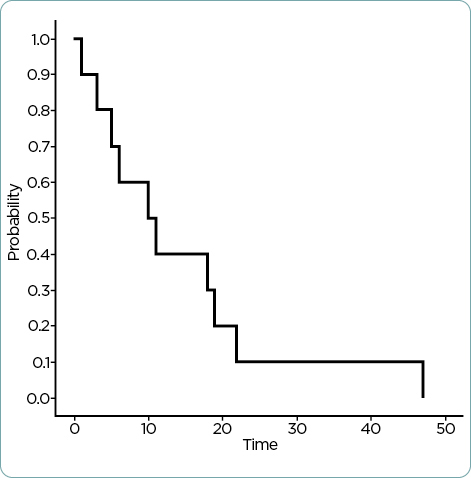
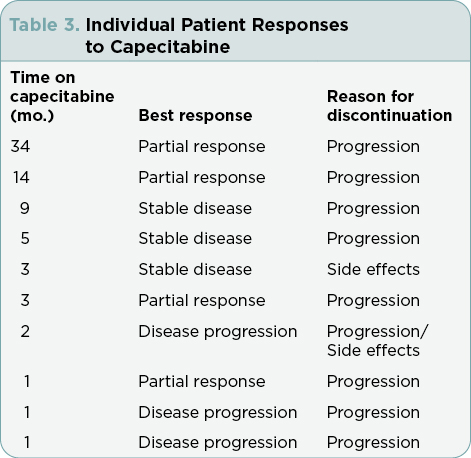
The median duration of time on therapy was 3 months (range 1–34 months). Reasons for discontinuation included disease progression (n = 8) and side effects (n = 2). Patients experienced cytopenias, palmar-plantar erythrodysesthesia, fatigue, rash, paresthesias, edema, nausea, and diarrhea (Table 2). Grading of these adverse effects ranged from mild to moderate and did not warrant discontinuation of therapy, only dose reduction. However, the two patients who discontinued therapy due to side effects experienced nontraditional adverse reactions to capecitabine, specifically, constipation and back pain in one patient, as well as weakness in the second patient. The median OS was 34.5 months (Figure 1), and the median survival on capecitabine was 10.5 months (Figure 2).
As reported in Table 3, the best treatment response was as follows: PR was seen in 4 of 10 (40%), SD in 3 of 10 (30%), and disease progression in 3 of 10 (20%). The CBR (CR + PR + SD) was 70%. One patient notably had prolonged clinical benefit from capecitabine and continued to be on treatment for 34 months total.
Discussion
In an unselected population of patients with HNSCC, capecitabine as salvage therapy resulted in a median survival of 7.3 months (Martinez-Trufero et al., 2010). In contrast, our study of patients with R/M HPV-OPC demonstrated a longer median survival of 10.5 months.
Patients with R/M HPV-OPC can live for years in the face of metastatic disease and can be candidates for multiple lines of therapy. Despite the highly selective and limited number of patients, a PR rate of 40% and a CBR of 70% in a heavily pretreated population is an interesting finding resulting from this study and may warrant further investigation. Although most patients were heavily pretreated, the tolerability of capecitabine was good and even allowed for extended treatment in some patients.
This study was limited by the small sample size and the retrospective nature of the data collection. The use of PET/CT scans without diagnostic CT for surveillance in many of the patients limited the resolution of the cross-sectional CT images and made assessment by RECIST impossible. Therefore, responses were delineated by investigator-assessed clinical response according to radiologic images. In addition, our patient cohort was limited to those who were able to travel to be followed and treated at our institution, which could bias our sample toward patients with a better performance status and survival. As all our patients were men, the efficacy and tolerance of salvage capecitabine for women with R/M HPV-OPC is unknown.
Given the potential signal of efficacy, future research endeavors should include conduct of a larger prospective study using capecitabine in patients with R/M HPV-OPC in comparison with other salvage treatment options and investigation into potential biomarkers of response.
Conclusion
Capecitabine showed promising activity in heavily pretreated patients with R/M HPV-OPC and is a potential option for salvage treatment. Further study is warranted to better define its efficacy and the ideal target population.
Disclosure
The authors have no conflicts of interest to disclose.
References
Brkic, F. F., Kadletz-Wanke, L., Kenner, L., Fureder, T., Jank, B., Brunner, M., & Heiduschka, G. (2021). An analysis of distant metastasis cases from HPV-associated oropharyngeal squamous cell carcinoma. Journal of Cranio-Maxillofacial Surgery, 49(4), 312–316. https://doi.org/10.1016/j.jcms.2021.01.012
Burtness, B., Harrington, K. J., Greil, R., Soulieres, D., Tahara, M., de Castro, G., Jr.,…Rischin, D., on behalf of the KEYNOTE-048 Investigators. (2019). Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet, 394(10212), 1915–1928. https://doi.org/10.1016/S0140-6736(19)32591-7
Centers for Disease Control and Prevention. (2023). HPV and oropharyngeal cancer. https://www.cdc.gov/cancer/hpv/basic_info/hpv_oropharyngeal.htm#:~:text=The%20vaccine%20protects%20against%20the,to%2012%2Dyear%2Dolds.
Martinez-Trufero, J., Isla, D., Adansa, J. C., Irigoyen, A., Hitt, R., Gil-Arnaiz, I.,…Cruz, J. J. (2010). Phase II study of capecitabine as palliative treatment for patients with recurrent and metastatic squamous head and neck cancer after previous platinum-based treatment. British Journal of Cancer, 102(12), 1687–1691. https://doi.org/10.1038/sj.bjc.6605697
National Comprehensive Cancer Network. (2022). NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers. V.1.2022. https://www.nccn.org/guidelines/nccn-guidelines/guidelines-detail?category=1&id=1437
Sacks, R., Law, J. Y., Zhu, H., Beg, M. S., Gerber, D. E., Sumer, B. D.,...Khan, S. A. (2020). Unique patterns of distant metastases in HPV-positive head and neck cancer. Oncology, 98(3), 179–185. https://doi.org/10.1159/000504651
Siegel, R. L., Miller, K. D., Wagle, N. K., & Jemal, A. (2023). Cancer Statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48. https://doi.org/10.3322/caac.21763
Van Dyne, E. A., Henley, S. J., Saraiya, M., Thomas, C. C., Markowitz, L. E., & Benard, V. B. (2018). Trends in Human Papillomavirus-Associated Cancers - United States, 1999-2015. Morbidity and Mortality Weekly Report, 67(33), 918–924. https://doi.org/10.15585/mmwr.mm6733a2
Wierzbicka, M., Klussmann, J. P., San Giorgi, M. R., Wuerdemann, N., & Dikkers, F. G. (2021). Oral and laryngeal HPV infection: Incidence, prevalence and risk factors, with special regard to concurrent infection in head, neck and genitals. Vaccine, 39(17), 2344–2350. https://doi.org/10.1016/j.vaccine.2021.03.047
Wisniewska-Jarosinska, M., Sliwinski, T., Kasznicki, J., Kaczmarczyk, D., Krupa, R., Bloch, K.,…Morawiec-Sztandera, A. (2011). Cytotoxicity and genotoxicity of capecitabine in head and neck cancer and normal cells. Molecular Biology Reports, 38(6), 3679–3688. https://doi.org/10.1007/s11033-010-0482-7
You, E. L., Henry, M., & Zeitouni, A. G. (2019). Human papillomavirus-associated oropharyngeal cancer: Review of current evidence and management. Current Oncology, 26(2), 119–123. https://doi.org/10.3747/co.26.4819