Meeting Abstract
JL407. Burden of Phlebotomy in Patients With Polycythemia Vera in the United States: A Preliminary Analysis of Baseline Data from the REVEAL Study
Brady L. Stein, MD, MHS, Northwestern University Feinberg School of Medicine, Chicago, IL, Ralph V. Boccia, MD, FACP, The Center for Cancer and Blood Disorders, Bethesda, MD, Alison R. Moliterno, MD, Johns Hopkins University School of Medicine, Baltimore, MD, Michael R. Grunwald, MD, Levine Cancer Institute, Carolinas HealthCare System, Charlotte, NC, Stephen T. Oh, MD, PhD, Washington University School of Medicine, St. Louis, MO, Ahmad B. Naim, MD, Incyte Corporation, Wilmington, DE, Dilan C. Paranagama, PhD, Incyte Corporation, Wilmington, DE, Hao Sun, MS, Incyte Corporation, Wilmington, DE, Shreekant V. Parasuraman, BPharm, PhD, Incyte Corporation, Wilmington, DE, Ruben A. Mesa, MD, FACP, Mayo Clinic Cancer Center, Scottsdale, AZ
 ABSTRACT
ABSTRACT
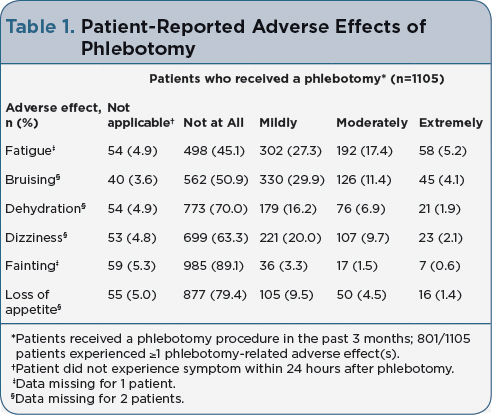
Background: Phlebotomy is often used to maintain hematocrit < 45% and prevent thrombotic events in patients with polycythemia vera (PV); however, phlebotomy procedures are inconvenient and/or poorly tolerated by some patients. Iron deficiency resulting from phlebotomy may be associated with burdensome symptoms, including fatigue, impaired cognitive function, and restless legs syndrome. The ongoing REVEAL study (NCT02252159) aims to describe contemporary demographics, disease burden, clinical management, and patient-reported outcomes among US patients with PV. This analysis presents the patient-reported burden of phlebotomy for currently enrolled patients (data cutoff: April 28, 2016). Methods: REVEAL is a prospective, observational study enrolling adults aged ≥18 years with PV under physician supervision. Physician assessments and patient-reported outcomes are being collected during a 36-month period. Phlebotomy-related burden in patients and their caregivers is evaluated using the 21-item phlebotomy burden questionnaire (PBQ-21), assessing phlebotomy frequency, time required for phlebotomy, practice setting, inconvenience, and phlebotomy-related adverse effects. Phlebotomy-related effects and bother/inconvenience (≤24 hours following the procedure) are graded from 1 (not at all) to 4 (extremely). Results: At data cutoff, 2307 patients were enrolled (planned enrollment, n=2500); 1105 patients completed PBQ-21 and had a phlebotomy within the previous 3 months. Median (range) age was 65 (22–95) years; the majority were male (61.5%) and white (91.9%). Median disease duration (PV diagnosis to enrollment) was 3.3 years. Mean (SD) number of phlebotomies was 2.2 (1.6), and total treatment time was 4.2 (5.1) hours per procedure. Employed patients (n=488) reported missing 4.9 (13.3) hours of work time per procedure. Phlebotomy was most often performed at an infusion or specialized cancer care center (32.5%) or at a physician’s office during a regular medical visit (30.0%). Most patients (72.5%) experienced ≥1 phlebotomy-related adverse effect(s); the most common are reported in Table 1. Many patients reported that phlebotomy procedures were moderately or extremely bothersome (18.0%), inconvenient (15.9%), painful or physically uncomfortable (16.1%), and inconvenient for family and friends (8.2%). Conclusions: PBQ-21 is a novel and valuable instrument to assess the effects of phlebotomy on patients and caregivers. Fatigue, bruising, dehydration, and dizziness resulting from phlebotomy procedures were reported in a considerable proportion of patients with PV. Additionally, these preliminary data suggest that phlebotomy treatment is associated with pain, discomfort, inconvenience, and notable time commitments in some patients. Implications: These findings provide physicians, patients, and caregivers with important insight into the burden of phlebotomy in patients with PV and may help guide individualized treatment decisions.
For access to the full length article, please
sign in.