Treatment With Carfilzomib: A Promising Future for Multiple Myeloma
From Mount Sinai Medical Center, New York, New York, and Winship Cancer Institute of Emory University, Atlanta, Georgia
Ms. Catamero has nothing to disclose. Ms. Gleason has acted as a consultant for Celgene Corporation.
Correspondence to: Charise Gleason, MSN, ANP, AOCNP®, Department of Hematology and Medical Oncology, Winship Cancer Institute of Emory University, 1365 Clifton Road NE, Atlanta, GA 30322. E-mail: charise.gleason@emoryhealthcare.org
J Adv Pract Oncol 2013;4(Suppl 1):33–43 | © 2013 Harborside Press®


ABSTRACT
The survival outlook for patients with multiple myeloma (MM) has improved over the past decade; however, even with access to newer therapies, all patients will eventually relapse and progress. Carfilzomib is a selective proteasome inhibitor and a promising MM treatment that was approved by the US Food and Drug Administration (FDA) in 2012. This review will highlight the future of carfilzomib by summarizing how the agent is being investigated in ongoing clinical trials. While standard carfilzomib (FDA-approved dose and schedule) is effective for MM treatment, clinical trials are investigating alternate dosing schemes of carfilzomib, including higher doses with longer infusion times and altered dosing schedules. Additionally, combination studies are elucidating important treatment options, both for patients who have relapsed and refractory MM and for patients who are newly diagnosed with MM. Drug combinations with carfilzomib have the potential to offer novel treatment options and improved overall survival. Finally, ongoing phase III randomized studies with carfilzomib will establish a broader representation of the many potential capabilities of the drug. The results of these trials will help to further define the role of carfilzomib in MM therapy, which may help to provide patients with improved overall survival and quality of life.
ARTICLE
T he outlook for patients with multiple myeloma (MM) has changed dramatically over the past decade, with the advent of new therapies that have led to improved survival rates (Kastritis et al., 2009; Kumar et al., 2008; Lonial, Mitsiades, & Richardson, 2011). Unfortunately, even with access to newer therapies, all patients will eventually relapse and progress (Kumar et al., 2012; Laubach et al., 2011). Treatment-related toxicities remain a concern. Advances in MM treatment will require regimens that balance efficacy and safety, and sequential regimens may be necessary (Mohty et al., 2012; van de Donk et al., 2011). The treatment of patients with relapsed and refractory MM (RR MM) is particularly challenging due to a combination of the disease itself and side effects of various treatments (Laubach et al., 2011; van de Donk et al., 2011).
With the advent of bortezomib (Velcade), the proteasome became a validated target in myeloma management, and combinations with other agents have proven successful in myeloma management (Nooka et al., 2013). Carfilzomib (Kyprolis) was granted accelerated approval by the US Food and Drug Administration (FDA) in 2012 for the treatment of patients with MM who have received at least two prior therapies, including bortezomib and an immunomodulatory drug (IMiD), and who have demonstrated disease progression on or within 60 days of completion of last therapy (Onyx Pharmaceuticals, 2012).
Single-agent carfilzomib, administered intravenously (IV) over 2 to 10 minutes at doses up to 27 mg/m2 (standard carfilzomib, i.e., the FDA-approved dose) has proven to be effective for patients with RR MM (Badros et al., 2013; Jagannath et al., 2012; Siegel et al., 2012; Vij et al., 2012a, 2012b). Carfilzomib is being investigated as a single agent when infused over a longer time, allowing for administration of higher doses, as well as in combination with other MM treatments in patients with both RR MM and newly diagnosed MM. This review summarizes the findings from these trials that aim to help define the present and future role of carfilzomib in the armamentarium of MM treatment. An overview of ongoing carfilzomib trials is illustrated in Table 1.
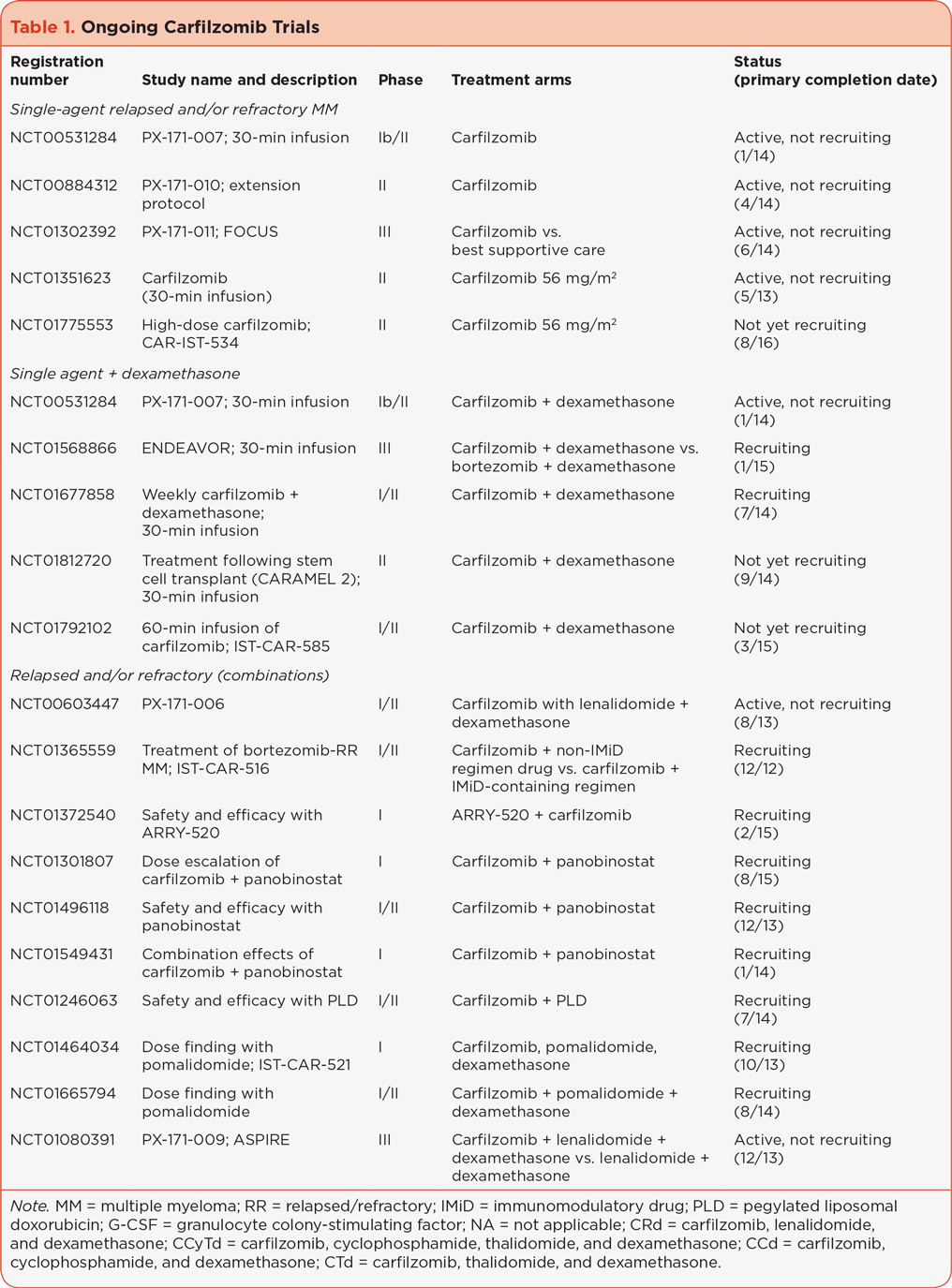

ALTERNATIVE DOSES AND SCHEDULES
While a dose of 20/27 mg/m2 of single-agent carfilzomib is effective in treating RR MM, alternate dosing schedules are being explored. Early clinical data suggested a carfilzomib dose response in a multivariate modeling analysis (Squifflet et al., 2011). Additionally, preclinical data showed lower maximum concentration (Cmax), equivalent proteasome inhibition, and reduced toxicity with longer infusion times of carfilzomib (Yang et al., 2011). These data led to testing a 30-minute infusion of carfilzomib in order to administer higher carfilzomib doses. PX-171-007 (referred to here as 007, NCT00531284) and NCT01351623 are single-agent, 30-minute infusion studies in patients with RR MM treated with carfilzomib from which promising response rates have been reported (Table 2).
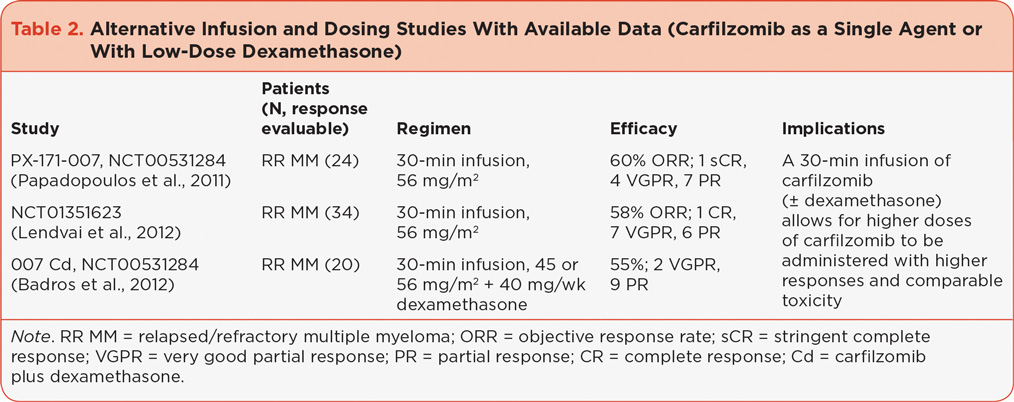
The 007 study established a maximum tolerated dose (MTD) of 56 mg/m2, at which dose there was an overall response rate (ORR) of 60% (Papadopoulos et al., 2011). The majority of adverse events (AEs) at this dose were grade 1 and 2 with the exception of thrombocytopenia, anemia, and hypertension (grade ≥ 3 in 38%, 21%, and 13% of patients, respectively), and 21% of patients required a dose reduction. In the other 30-minute single-agent infusion study, ORR in patients who completed four cycles of therapy was 58% (50% in the overall population) and median progression-free survival (PFS) was 4.6 months (Lendvai et al., 2012). The most common treatment-related, nonhematologic grade 3/4 AEs included hypertension (21%), lung infection (18%), and pulmonary edema (9%), and 35% of patients required a dose reduction.
Additional ongoing studies investigating a higher dose of carfilzomib through longer infusion times include CARAMEL 2 (NCT01812720), which is investigating a 30-minute carfilzomib infusion following stem cell transplantation, and a phase I/II study (NCT01792102) investigating a 60-minute infusion of carfilzomib 56 mg/m2. Alternate dosing schedules are also being investigated. The CHAMPION-1 study (NCT01677858), which is currently recruiting patients, is specifically investigating once-weekly dosing of carfilzomib.
COMBINATION STUDIES
Due to the heterogeneity of MM, combination therapy has been demonstrated as an important treatment option. Many studies are under way investigating combinations with carfilzomib in patients with RR MM (Table 3) and newly diagnosed MM (Table 4).
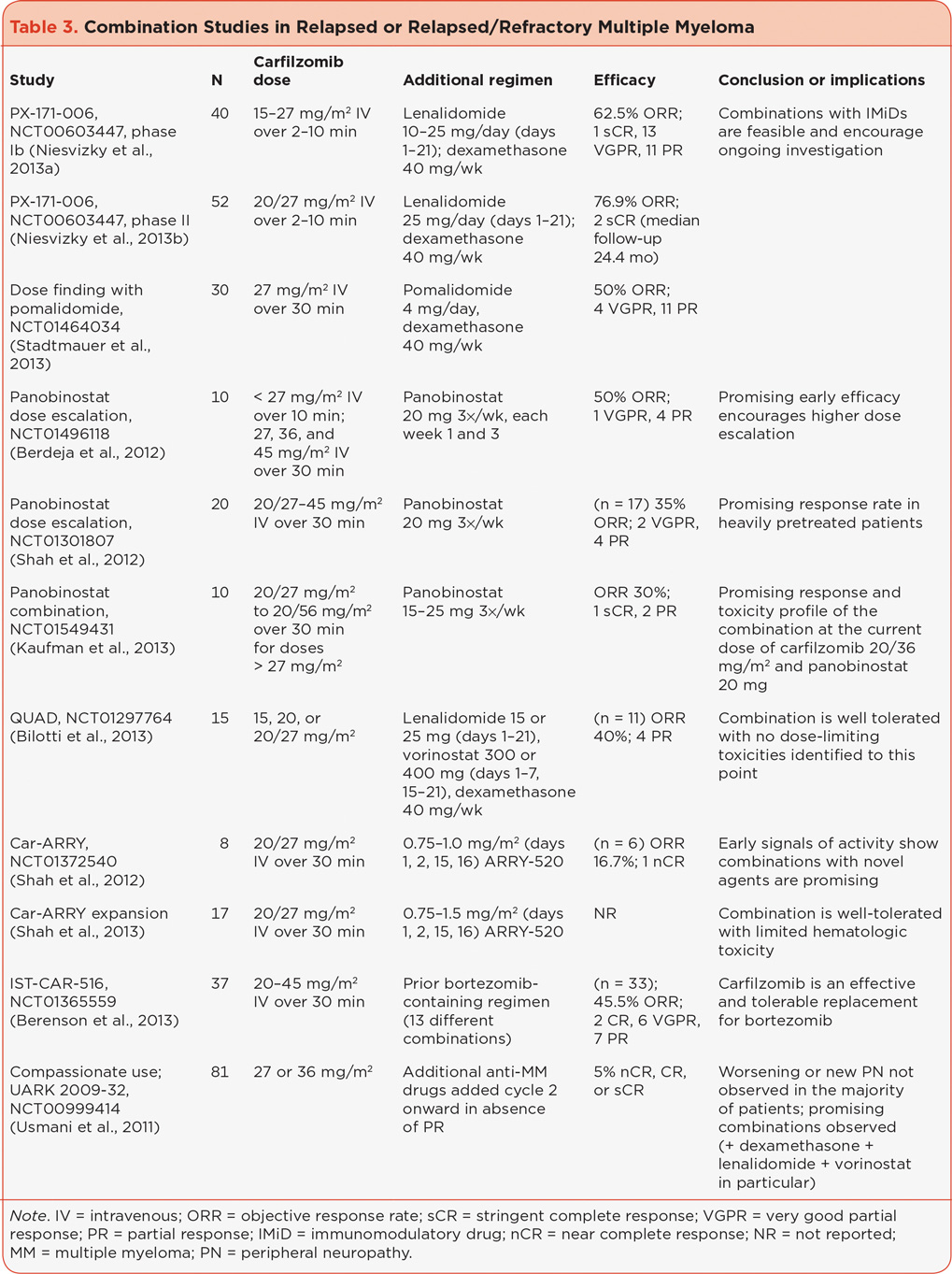

Combinations in Patients With Relapsed or Relapsed Refractory MM
Treatment for patients with RR MM is complicated because the treatment depends on both disease-related factors, i.e., quality and duration of response to other treatments, aggressiveness of the disease, and patient-related factors, i.e., preexisting toxicity from previous therapy, age, performance status, and comorbidities (Mohty et al., 2012). Patients at high risk for disease are generally treated with combination regimens for maximal response (Laubach et al., 2011).
Current studies have reported promising results when combining carfilzomib with IMiDs lenalidomide (Revlimid) or pomalidomide (Pomalyst). PX-171-006 (NCT00603447) investigated the combination of carfilzomib with lenalidomide and dexamethasone in a phase Ib (Niesvizky et al., 2013a) and phase II (Niesvizky et al., 2013b) trial of patients with relapsed or progressive MM. In the 40 patients evaluable for response across the dose-escalation groups (phase Ib, in which the carfilzomib doses ranged from 15 to 27 mg/m2), the ORR was 62.5% with a duration of response (DOR) for patients with ≥ partial response (PR) of 11.8 months and overall PFS of 10.2 months (Niesvizky et al., 2013a). The combination had a tolerable safety profile, and the MTD was not reached. Therefore, the maximum planned dose (MPD) of 27 mg/m2 was expanded in the phase II portion in 52 patients in whom an ORR of 76.9% was reported with a median PFS of 15.4 months (Niesvizky et al., 2013b). The treatment was well tolerated with discontinuations due to an AE reported for 15% of patients.
Trials NCT01665794 and NCT01464034 (Stadtmauer et al., 2013) are dose-finding studies combining carfilzomib with pomalidomide. Results are available from the latter study in which an MTD of 20/27 mg/m2 carfilzomib, 4 mg pomalidomide, and 40 mg dexamethasone was established and an ORR of 50% was reached (Stadtmauer et al., 2013). The combination was well tolerated with limited grade 3/4 toxicities.
Additionally, three studies combining the histone deacetylase (HDAC) inhibitor panobinostat with carfilzomib are in progress (NCT01496118, NCT01301807, and NCT01549431); preliminary results demonstrate promising response rates with a tolerable safety profile and no unexpected toxicities and merit the investigation of higher doses (Berdeja et al., 2012; Kaufman et al., 2013; Shah et al., 2012a). QUAD (NCT01297764) is a four-drug combination of carfilzomib, lenalidomide, the HDAC inhibitor vorinostat (Zolinza), and dexamethasone designed to determine the MTD and safety/tolerability of the combination. No dose-limiting toxicities (DLTs) have yet been reported at the current combination of carfilzomib 20/27 mg/m2, lenalidomide 25 mg, vorinostat 300 mg, and dexamethasone 40 mg, with a promising preliminary response rate and manageable safety profile (Bilotti et al., 2013).
When carfilzomib was combined with ARRY-520 (NCT01372540), a novel kinesin spindle protein inhibitor (Shah et al., 2012b), the combination was well tolerated and showed signs of activity. Updated results report accrual ongoing at the final cohort of full-dose ARRY-520 of 1.5 mg/m2 and 20/27 mg/m2 carfilzomib with 1 DLT in that cohort and 9/17 patients overall still on the study, with no patients discontinuing therapy due to toxicity (Shah et al., 2013). Other combination studies with no reported results to date include combination with pegylated liposomal doxorubicin (NCT01246063), a carfilzomib conditioning study with high-dose melphalan (NCT01690143), and a bone marrow study combining filgrastim plus carfilzomib or bortezomib (NCT01537861).
Some studies are investigating carfilzomib in multiple combinations simultaneously. Bortezomib has been replaced with carfilzomib in a phase I/II study in bortezomib-refractory patients who had received bortezomib in a combination regimen with or without an IMiD agent (NCT01365559; Berenson et al., 2012). Updated results for this study indicate that an MTD has been reached for at least one regimen (carfilzomib 45 mg/m2 plus ascorbic acid plus cyclophosphamide), and clinical benefit was seen in 70% of patients with 6% complete response (CR) and 18% very good partial response (VGPR; Berenson et al., 2013). The combinations are well tolerated with the most common grade ≥ 3 AEs being all hematologic, including lymphopenia (35%), thrombocytopenia (19%), neutropenia (11%), and anemia (8%). Efficacy with various combinations also was seen in the carfilzomib compassionate use program UARK 2009-32 (NCT00999414), where anti-MM drugs were added to carfilzomib in cycle 2 in the absence of a PR, resulting in the identification of combinations with encouraging activity (Usmani et al., 2011).
Combinations in Patients With Newly Diagnosed MM
Although carfilzomib is currently approved for patients with RR MM, several studies using carfilzomib in the up-front setting suggest the use of carfilzomib in first-line MM treatment. A phase I/II study combined carfilzomib with lenalidomide and dexamethasone in patients with newly diagnosed MM eligible or ineligible for transplant (CRd, NCT01029054; Jakubowiak et al., 2012). Updated results of this study after a median of 22 cycles show an ORR of 98%, ≥ VGPR of 87% with a 24-month PFS of 94%, and overall survival (OS) at 98%; extended treatment continued to be well-tolerated (Jakubowiak et al., 2013a). In a subset of patients aged ≥ 65 years (n = 23), an ORR of 100% and ≥ VGPR of 87% were reported with AEs rates comparable to overall data with trends of higher thrombocytopenia, neutropenia, and hyperglycemia (Jakubowiak et al., 2013b).
Another study of the CRd combination (NCT01402284) was unique in that transplant-eligible patients defaulted to delayed autologous stem cell transplantation (ASCT) and minimal residual disease analyses were used to determine molecular response, illustrating that CRd induces rapid and deep remissions with tolerable side effects (Korde et al., 2013). Based on the data from these 2 CRd studies, carfilzomib + lenalidomide + dexamethasone was added as a Category 2A option for primary transplant-eligible patients according to National Comprehensive Cancer Network (NCCN) guidelines (NCCN, 2013).
Carfilzomib is rapidly effective when combined with the first-generation immunomodulatory agent, thalidomide (Thalomid). CARTHADEX (NTR2422) is a phase I/II dose-escalation trial to investigate carfilzomib combined with thalidomide and dexamethasone (CTd) for induction and consolidation in transplant-eligible patients with newly diagnosed MM (Sonneveld et al., 2012). In cohort 1 (n = 40, carfilzomib 27 mg/m2) the ORR was 88% with ≥ CR in 18% of 35 patients who completed induction and an ORR of 90% with ≥ CR in 35% of the 17 patients who completed consolidation. Results for this trial show the treatment was well tolerated with no hematologic toxicities observed and grade 3/4 nonhematologic toxicities including tumor lysis syndrome (5%), deep vein thrombosis (10%), gastrointestinal symptoms (5%), and skin rash (8%). The results also indicate response can be enhanced with consolidation treatment with carfilzomib.
Additional treatment plans include four-drug combinations, alternate carfilzomib dosing schemes, and sequential treatment. The addition of cyclophosphamide to carfilzomib, thalidomide, and dexamethasone indicates that four-drug combinations can be a useful treatment plan for carfilzomib. CYCLONE (NCT01057225) is a phase I/II trial to investigate carfilzomib combined with cyclophosphamide, thalidomide, and dexamethasone in patients with newly diagnosed MM intended to proceed to ASCT. In 27 response-evaluable patients, ORR was 96% with 29% CR, 46% VGPR, and 21% PR (Mikhael et al., 2012).
CARMYSAP (NCT01279694) is a phase I/II trial investigating the alternate dosing scheme of carfilzomib in 42-day cycles for 9 cycles followed by the combination of carfilzomib with melphalan and prednisone (CMP) in elderly patients not eligible for ASCT. An MTD of carfilzomib 20/36 mg/m2 was reached in the initial phase, and this dose was expanded to an additional 45 patients in phase II for a total of 69 patients, which resulted in an ORR of 89% with > VGPR of 51% (Moreau et al., 2013). The combination was well tolerated without peripheral neuropathy (PN) ≥ grade 2. Additionally, carfilzomib as maintenance treatment is being investigated in a phase II study (NCT01346787) evaluating carfilzomib combined with cyclophosphamide and dexamethasone (CCd) for 9 cycles followed by carfilzomib maintenance every 28 days until progression. In 41 response-evaluable patients, the ORR was 93% with stringent CR of 12% and ≥ CR/near CR of 46%. Responses were rapid (median time to PR, 1 month; median time to CR, 2 months) and improved with the duration of treatment reaching 100% ≥ PR and 77% ≥ VGPR after 9 cycles (Bringhen et al., 2013; Palumbo et al., 2013). The combination was well tolerated with 16% patients requiring a carfilzomib dose reduction and 11% discontinuing treatment due to an AE.
Car-BiRD (NCT01559935) is a phase II study evaluating carfilzomib as a part of sequential treatment. Carfilzomib treatment is followed by clarithromycin, lenalidomide, and dexamethasone and then lenalidomide maintenance treatment. Other studies with no results reported to date include a phase I study combining carfilzomib with cyclophosphamide and dexamethasone prior to ASCT (NCT01660750) and a phase II study combining carfilzomib with lenalidomide and dexamethasone before and after ASCT (NCT01816971).
RANDOMIZED STUDIES
Randomized studies with carfilzomib will establish a more broad representation of the drug’s capabilities. FOCUS (NCT01302392) is a phase III, randomized, open-label, international, multicenter study investigating standard carfilzomib vs. low-dose corticosteroids and optional cyclophosphamide in patients with RR MM (Hajek et al., 2012). The primary endpoint is OS, with findings from the study expected to facilitate potential European Medicines Agency approval. The phase II 006 trial combining carfilzomib with lenalidomide informed ASPIRE (NCT01080391), a phase III, randomized, open-label, multicenter trial of standard carfilzomib combined with lenalidomide and dexamethasone vs. lenalidomide and dexamethasone alone in patients with relapsed or progressive MM (Moreau et al., 2011).
An amendment of the 007 trial that combined 30-minute infusion of 45 or 56 mg/m2 carfilzomib with low-dose dexamethasone led to ENDEAVOR (NCT01568866), a phase III, head-to-head trial of high-dose carfilzomib (56 mg/m2) infused over 30 minutes combined with low-dose dexamethasone vs. standard bortezomib (1.3 mg/m2) combined with low-dose dexamethasone in patients with relapsed or progressive MM.
CARMYSAP, combining carfilzomib with melphalan, informed CLARION (NCT01818752), a phase III multicenter, open-label, randomized study in transplant-ineligible patients with newly diagnosed MM. Carfilzomib 36 mg/m2 is administered over 30 to 60 minutes with melphalan and prednisone compared with standard bortezomib (IV or SC) with melphalan and prednisone. These randomized phase III trials are meant to inform the wider use of carfilzomib in MM.
CONCLUSION AND FUTURE DIRECTIONS
Survival outcomes are very poor when a patient with MM develops relapsed and/or refractory disease and treatment becomes more challenging. Significant progress has been made in the management of myeloma over the past 10 years with the development of new therapies including lenalidomide and bortezomib. Carfilzomib is a selective proteasome inhibitor with significant activity in RR MM at the FDA-approved dose and schedule. Carfilzomib has demonstrated robust and durable antitumor activity with evidence of a dose-response relationship in this patient population with a well-tolerated side-effect profile characterized by low rates of PN. Data from clinical trials evaluating efficacy and safety of carfilzomib alone and in combination with other agents in various stages of disease will help define the MM treatment paradigm. Clinical trials continue to focus on single-agent, combination, consolidation, and maintenance treatment in the up-front and relapsed setting. Combination regimens are associated with higher response rates, and ongoing trials are concentrating on combinations with IMiDs (lenalidomide and pomalidomide), HDAC inhibitors (panobinostat and vorinostat), and cytotoxic therapies (PLD, melphalan, and cyclophosphamide). These drug combinations have the potential to offer additional treatment options and further improve OS.
As carfilzomib data continue to evolve and demonstrate promise, the results of ongoing phase II and multiple phase III trials will help to further define the role of carfilzomib in MM therapy and will help establish the best dose, schedule, and supportive care in these patients. When used with appropriate consideration, carfilzomib may help to provide patients with an improved overall survival and quality of life.
ACKNOWLEDGMENT
The authors would like to thank Melissa Kirk, PhD (Fishawack Communications), for medical writing and editorial assistance, supported by Onyx Pharmaceuticals, Inc.
DISCLOSURE
Ms. Catamero has nothing to disclose. Ms. Gleason has acted as a consultant for Celgene Corporation.
REFERENCES
Badros, A., Papadopoulos, K., Zojwalla, N., Lee, J., & Siegel, D. (2012). A phase Ib study of 30-minute infusion carfilzomib 20/45 and 20/56 mg/m2 plus 40 mg weekly dexamethasone in patients with relapsed and/or refractory (R/R) multiple myeloma [Abstract 4036]. Blood (ASH Annual Meeting Abstracts), 120.
Badros, A. Z., Vij, R., Martin, T., Zonder, J. A., Kunkel, L., Wang, Z.,...Niesvizky, R. (2013). Carfilzomib in multiple myeloma patients with renal impairment: Pharmacokinetics and safety. Leukemia, 27, 1707–1714. http://dx.doi.org/10.1038/leu.2013.29
Berdeja, J., Hart, L., Lamar, R., Murphy, P., Morgan, S., & Flinn, I. (2012). Phase I/II study of panobinostat and carfilzomib in patients (pts) with relapsed or refractory multiple myeloma (MM), interim phase I safety analysis [Abstract 4048]. Blood (ASH Annual Meeting Abstracts), 120.
Berenson, J., Hilger, J., Dichmann, R., Patel-Donnelly, D., Boccia, R., Bessudo, A.,…Vescio, R. (2013). Results of a phase I/II study (NCT01365559) of carfilzomib (CFZ) replacing bortezomib (BTZ) in BTZ-containing regimens for BTZ-treated patients (pts) with relapsed and refractory multiple myeloma (MM) [Abstract 8599]. Journal of Clinical Oncology, 31(suppl).
Berenson, J. R., Hilger, J. D., Dichmann, R., Patel-Donnelly, D., Boccia, R. V., Bessudo, A.,…Vescio, R. (2012). A phase 1/2 study of carfilzomib as a replacement for bortezomib for multiple myeloma (MM) patients (pts) refractory to a bortezomib-containing combination regimen [Abstract 4063]. Blood (ASH Annual Meeting Abstracts), 120.
Bilotti, E., Vesole, D., Richter, J., McNeill, A., Anand, P., Bednarz, U.,…Siegel, D. (2013). Preliminary results of a phase I/II study of carfilzomib, lenalidomide, vorinostat and dexamethasone (QUAD) in relapsed and/or refractory multiple myeloma (MM) [Abstract P774]. Haematologica, 98(suppl 1).
Bringhen, S., Federica, C., Petrucci, M., Gay, F., Federico, V., Conticello, C.,…Palumbo, A. (2013). Carfilzomib, cyclophosphamide and dexamethasone (CCD) for newly diagnosed multiple myeloma (MM) patients: Initial results of a multicenter, open label phase II study [Abstract S578] Haematologica, 98(suppl 1).
Hájek, R., Bryce, R., Ro, S., Klencke, B., & Ludwig, H. (2012). Design and rationale of FOCUS (PX-171-011): A randomized, open-label, phase 3 study of carfilzomib vs.. best supportive care regimen in patients with relapsed and refractory multiple myeloma (R/R MM). BMC Cancer, 12, 415. http://dx.doi.org/10.1186/1471-2407-12-415
Jagannath, S., Vij, R., Stewart, A. K., Trudel, S., Jakubowiak, A. J., Reiman, T.,...Siegel, D. S. (2012). An open-label single-arm pilot phase II study (PX-171-003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. Clinical Lymphoma, Myeloma, & Leukemia, 12(5), 310–318. http://dx.doi.org/10.1016/j.clml.2012.08.003
Jakubowiak, A., Dytfeld, D., Griffith, K., Jasielec, J., McDonnell, K., Lebovic, D.,…Kaminski, M. (2013a). Treatment outcome with the combination of carfilzomib, lenalidomide, and low-dose dexamethasone (CRd) for newly diagnosed multiple myeloma (NDMM) after extended follow-up [Abstract 8543]. Journal of Clinical Oncology (Meeting Abstracts), 31(suppl).
Jakubowiak, A., Dytfeld, D., Jasielec, J., Griffith, K., Lebovic, D., Vesole, D.,...Vij, R. (2013b). Carfilzomib, lenalidomide, low-dose dexamethasone (CRd) in elderly patients with newly diagnosed multiple myeloma (NDMM) [Abstract O-10]. Clinical Lymphoma, Myeloma, & Leukemia, 13(suppl 1).
Jakubowiak, A. J., Dytfeld, D., Griffith, K. A., Lebovic, D., Vesole, D. H., Jagannath, S.,…Vij, R. (2012). A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood, 120(9), 1801–1809. http://dx.doi.org/10.1182/blood-2012-04-422683
Kastritis, E., Zervas, K., Symeonidis, A., Terpos, E., Delimbassi, S., Anagnostopoulos, N.,...Dimopoulos, M. A. (2009). Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): An analysis of the Greek Myeloma Study Group (GMSG). Leukemia, 23(6), 1152-1157. http://dx.doi.org/10.1038/leu.2008.402
Kaufman, J., Zimmerman, T., Jakubowiak, A., Rosenbaum, C., Lewis, C., Harvey, R.,...Lonial, S. (2013). Phase I study of the combination of carfilzomib and panobinostat for patients with relapsed and refractory myeloma: A multicenter MMRC clinical trial [Abstract P771]. Haematologica, 98(suppl 1).
Korde, N., Zingone, A., Kwok, M., Manasanch, E., Wu, P., Tageja, N.,...Landgren, O. (2013). Phase II clinical and correlative study of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended dosing (CRD-R) in newly diagnosed multiple myeloma (MM) patients [Abstract P228]. Haematologica, 98(suppl 1).
Kumar, S. K., Lee, J. H., Lahuerta, J. J., Morgan, G., Richardson, P. G., Crowley, J.,...Durie, B. G. (2012). Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter International Myeloma Working Group study. Leukemia, 26(1), 149–157. http://dx.doi.org/10.1038/leu.2011.196
Kumar, S. K., Rajkumar, S. V., Dispenzieri, A., Lacy, M. Q., Hayman, S. R., Buadi, F. K.,...Gertz, M. A. (2008). Improved survival in multiple myeloma and the impact of novel therapies. Blood, 111(5), 2516–2520. http://dx.doi.org/10.1182/blood-2007-10-116129
Laubach, J. P., Mitsiades, C. S., Mahindra, A., Luskin, M. R., Rosenblatt, J., Ghobrial, I. M.,...Richardson, P. G. (2011). Management of relapsed and relapsed/refractory multiple myeloma. Journal of the National Comprehensive Cancer Network, 9(10), 1209–1216.
Lendvai, N., Landau, H., Lesokhin, A., Tsakos, I., Koehne, G., Chung, D.,...Giralt, S. (2012). Phase II study of infusional carfilzomib in patients with relapsed or refractory multiple myeloma [Abstract 947]. Blood (ASH Annual Meeting Abstracts), 120.
Lonial, S., Mitsiades, C. S., & Richardson, P. G. (2011). Treatment options for relapsed and refractory multiple myeloma. Clinical Cancer Research, 17(6), 1264–1277. http://dx.doi.org/10.1158/1078-0432.CCR-10-1805
Mikhael, J., Reeder, C., Libby, E., Costa, M., Bergsagel, P., Buadi, F.,...Stewart, A. (2012). Results from the phase II dose expansion of cyclophosphamide, carfilzomib, thalidomide and dexamethasone (CYCLONE) in patients with newly diagnosed multiple myeloma [Abstract 445]. Blood (ASH Annual Meeting Abstracts), 120.
Mohty, B., El-Cheikh, J., Yakoub-Agha, I., Avet-Loiseau, H., Moreau, P., & Mohty, M. (2012). Treatment strategies in relapsed and refractory multiple myeloma: A focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia, 26(1), 73–85. http://dx.doi.org/10.1038/leu.2011.310
Moreau, P., Kolb, B., Hulin, C., Caillot, D., Benboubker, L., Tiab, M.,...Facon, T. (2013). CMP—carfilzomib (CFZ) plus melphalan-prednisone (MP)—in elderly patients (pts) with newly diagnosed multiple myeloma (NDMM): Results of a phase (PH) I/II trial [Abstract P224]. Haematologica, 98(suppl 1).
Moreau, P., Palumbo, A. P., Stewart, A. K., Rajkumar, V., Jakubowiak, A. J., Halka, K.,...Siegel, D. S. D. (2011). A randomized, multicenter, phase (Ph) III study comparing carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (Dex) to LEN and Dex in patients (Pts) with relapsed multiple myeloma (MM) [Abstract TPS225]. Journal of Clinical Oncology (Meeting Abstracts), 29(15 suppl).
National Comprehensive Cancer Network. (2013). NCCN Clinical Practice Guidelines in Oncology: Multiple myeloma, version 1.2013.
Niesvizky, R., Martin, T. G., 3rd, Bensinger, W. I., Alsina, M., Siegel, D. S., Kunkel, L. A.,...Wang, M. (2013a). Phase 1b dose-escalation study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clinical Cancer Research, 19, 2248–2256. http://dx.doi.org/10.1158/1078-0432.CCR-12-3352
Niesvizky, R., Wang, M., Martin, T., Bensinger, W., Alsina, M., Siegel, D., & Kavalerchik, E. (2013b). Final results from the phase 1b/2 study (PX-171-006) of carfilzomib (CFZ) in combination with lenalidomide and low-dose dexamethasone (CRD) for patients with relapsed or progressive multiple myeloma [Abstract S577]. Haematologica, 98(suppl1).
Nooka, A., Gleason, C., Casbourne, D., & Lonial, S. (2013). Relapsed and refractory lymphoid neoplasms and multiple myeloma with a focus on carfilzomib. Biologics, 7, 13–32. http://dx.doi.org/10.2147/BTT.S24580
Onyx Pharmaceuticals. (2012). Kyprolis (carfilzomib) package insert. Retrieved from http://www.kyprolis.com/prescribing-information
Palumbo, A., Bringhen, S., Petrucci, M., Oliva, S., Finsinger, P., Conticello, C.,...Boccadoro, M. (2013). A phase II trial of carfilzomib, cyclophosphamide and dexamethasone (CCd) for newly diagnosed multiple myeloma patients [Abstract P-145]. Clinical Lymphoma, Myeloma, and Leukemia, 13(suppl 1).
Papadopoulos, K. P., Lee, P., Singhal, S., Holahan, J. R., Vesole, D. H., Rosen, S. T.,...Siegel, D. S. (2011). A phase 1b/2 study of prolonged infusion carfilzomib in patients with relapsed and/or refractory (R/R) multiple myeloma: updated efficacy and tolerability from the completed 20/56 mg/m2 expansion cohort of PX-171-007 [Abstract 2930]. Blood (ASH Annual Meeting Abstracts), 118(21).
Redic, K. (2013). Carfilzomib: A novel agent for multiple myeloma. Journal of Pharmacy and Pharmacology, 65, 1095–1238. http://dx.doi.org/10.1111/jphp.12072
Shah, J., Thomas, S., Weber, D., Wang, M., Alexanian, M., Qazilbash, M.,...Orlowski, R. (2012). Phase 1/1b study of the efficacy and safety of the combination of panobinostat + carfilzomib in patients with relapsed and/or refractory multiple myeloma [Abstract 4081]. Blood (ASH Annual Meeting Abstracts), 120.
Shah, J., Thomas, S., Weber, D., Wang, M., & Orlowski, R. (2013). Phase I study of the novel kinesin spindle protein inhibitor arry-520 + carfilzomib (CAR) in patients with relapsed and/or refractory multiple myeloma (RRMM) [Abstract S579]. Haematologica, 98(suppl 1).
Shah, J., Weber, D., Thomas, S., Alexanian, R., Wang, M., Qazilbash, M.,...Orlowski, R. (2012). Phase I study of the novel kinesin spindle protein inhibitor ARRY-520 + carfilzomib in patients with relapsed and/or refractory multiple myeloma [Abstract 4082]. Blood (ASH Annual Meeting Abstracts), 120.
Siegel, D., Martin, T., Wang, M., Vij, R., Jakubowiak, A., Lonial, S.,...Jagannath, S. (2012). A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood, 120(14), 2817–2825. http://dx.doi.org/10.1182/blood-2012-05-425934
Sonneveld, P., Asselbergs, E., Zweegman, S., Van der Holt, B., Kersten, M., Vellenga, E.,...Lokhorst, H. (2012). Carfilzomib combined with thalidomide and dexamethasone (CTD) is an highly effective induction and consolidation treatment in newly diagnosed patients with multiple myeloma (MM) who are transplant candidate [Abstract 333]. Blood (ASH Annual Meeting Abstracts), 120.
Squifflet, P., Michiels, S., Siegel, D. S., Vij, R., Ro, S., & Buyse, M. E. (2011). Multivariate modelling reveals evidence of a dose-response relationship in phase II studies of single-agent carfilzomib [Abstract 1877]. Blood (ASH Annual Meeting Abstracts), 118(21).
Stadtmauer, E., Shah, J., Abonour, R., Cohen, A., Bensinger, W., Gasparetto, C.,...Durie, B. (2013). Carfilzomib, pomalidomide and dexamethasone (CPomd) for relapsed/refractory multiple myeloma (RRMM): A phase I/II trial. Clinical Lymphoma, Myeloma, and Leukemia, 13(suppl 1), P250.
Usmani, S., Szymonifka, J., Sexton, R., Panozzo, S., Nair, B., Waheed, S.,...Barlogie, B. (2011). Phase II study of carfilzomib (CFZ) combined with other anti-myeloma agents in relapsed-refractory multiple myeloma (RRMM)—Updates on the UARK compassionate use protocol [Abstract 2947]. Blood (ASH Annual Meeting Abstracts), 118(21).
van de Donk, N. W., Lokhorst, H. M., Dimopoulos, M., Cavo, M., Morgan, G., Einsele, H.,...Palumbo, A. (2011). Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treatment Reviews, 37(4), 266–283. http://dx.doi.org/10.1016/j.ctrv.2010.08.008
Vij, R., Siegel, D., Jagannath, S., Jakubowiak, A., Stewart, A., McDonagh, K.,...Wang, M. (2012a). An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. British Journal of Haematology, 158(6), 739–748. http://dx.doi.org/10.1111/j.1365-2141.2012.09232.x
Vij, R., Wang, M., Kaufman, J. L., Lonial, S., Jakubowiak, A. J., Stewart, A. K.,...Siegel, D. S. (2012b). An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood, 119(24), 5661–5670. http://dx.doi.org/10.1182/blood-2012-03-414359
Yang, J., Wang, Z., Fang, Y., Jiang, J., Zhao, F., Wong, H.,...Kirk, C. J. (2011). Pharmacokinetics, pharmacodynamics, metabolism, distribution, and excretion of carfilzomib in rats. Drug Metabolism and Disposition, 39(10), 1873–1882. http://dx.doi.org/10.1124/dmd.111.039164