Relapsed or Relapsed/Refractory Multiple Myeloma
From The University of Arizona Cancer Center, Tucson, Arizona
Ms. Kurtin has acted as a consultant for Onyx, Celgene, and Millennium.
Correspondence to: Sandra E. Kurtin, RN, MS, AOCN®, ANP-C, The University of Arizona Cancer Center, 3838 North Campbell Avenue, Tucson, AZ 85719. E-mail: sandra.kurtin@uahealth.com
J Adv Pract Oncol 2013;4(Suppl 1):5–14 | © 2013 Harborside Press®


ABSTRACT
Multiple myeloma (MM) is a malignant plasma cell disorder with potential secondary organ effects including renal, bone, and bone marrow effects as well as neurologic and immune dysfunction. Diagnostic evaluation of MM includes laboratory and radiologic studies along with bone marrow biopsy to confirm diagnosis. Multiple myeloma is a clonal plasma cell malignancy that results from complex interactions between malignant progenitor cells, bone marrow stromal cells, and the bone marrow microenvironment. Multiple myeloma is clinically and pathologically heterogeneous, which results in variability in treatment response and survival. The disease trajectory varies for each patient, but relapses are inevitable and many patients become refractory to treatments. Management of relapsed and refractory (RR) MM requires careful evaluation of individual patient characteristics and the course of the disease. When determining treatment options for patients with RR MM, comorbidities, the frailty and vulnerability of the patient, and the specific adverse event profile associated with each treatment should be considered, as well as the patient's goals. The goal of therapy for patients with RR MM is to achieve disease control with acceptable toxicity and quality of life, which may be accomplished with novel agents, most likely in combination regimens. The integration of these novel agents into the treatment paradigm has shifted the perception of MM from incurable to a disease that may be considered chronic in the near future with a hope for long-term survival and maintained quality of life.
ARTICLE
Multiple myeloma (MM) encompasses a heterogeneous group of malignant plasma cell disorders characterized by excess paraprotein secretion, secondary organ effects on the kidneys and bone, and neurologic, immune, and bone marrow dysfunction (Pingali, Haddad, & Saad, 2012; Raab, Podar, Breitkreutz, & Richardson, 2009). According to the American Cancer Society (ACS; 2013), approximately 22,350 new cases of MM are projected to occur in 2013 (12,440 males, 9,910 females) with 10,710 deaths (6,070 males, 4,640 females), with the average age at diagnosis being 69 years (National Cancer Institute, 2013). Risk factors for MM include advanced age, male gender, obesity, and African American descent (ACS, 2013; Perrotta et al., 2013). An increased incidence of myeloma is present in persons who have been exposed to chemicals, including pesticides, arsenic, cadmium, lead, and various cleaning solutions (Perrotta et al., 2013).
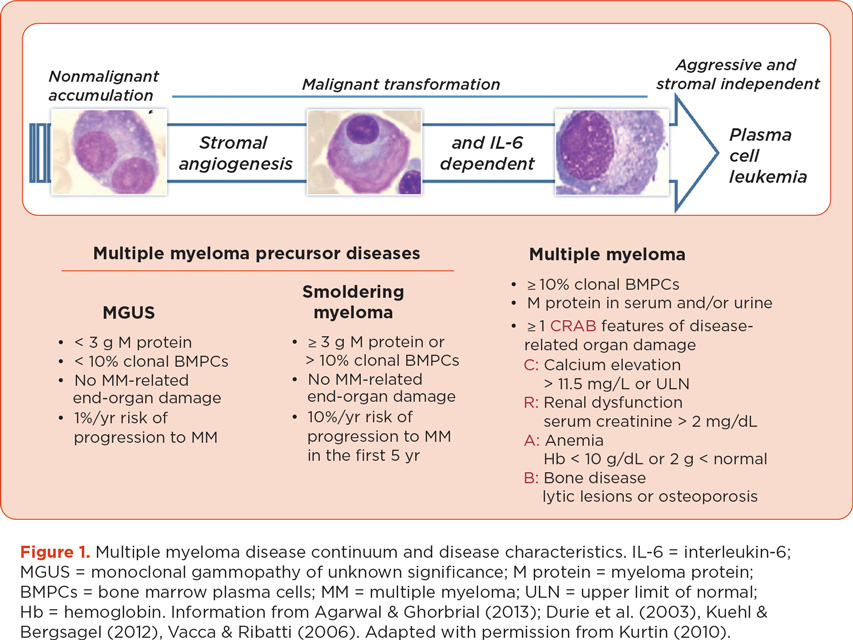
The initial diagnostic evaluation of MM includes both laboratory and radiologic studies to confirm the diagnosis, determine the subtype and stage, and identify the need for immediate intervention (Kurtin, 2012; National Comprehensive Cancer Network [NCCN], 2013; Pingali et al., 2012). The diagnosis of MM is based on the level of M protein in the serum or urine, the percentage of plasma cells present in the bone marrow, and the presence or absence of end-organ damage; see Figure 1 (Dimopoulos & Terpos, 2010; Durie et al., 2006; Kuehl & Bergsagel, 2012). Treatment is indicated for patients with MM-related end-organ dysfunction, commonly described by the CRAB criteria (hypercalcemia, renal impairment, anemia, and bone disease).
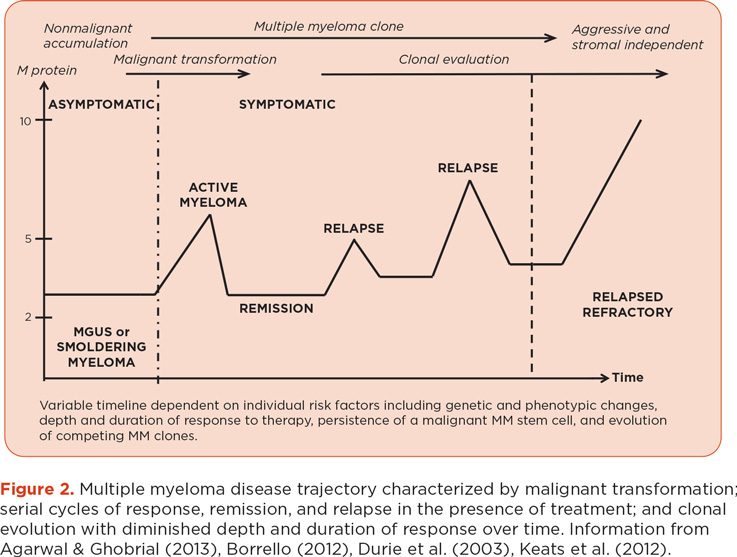
The primary goal of treatment for MM is to achieve an early, deep, and durable response with an acceptable level of toxicity. Achieving a durable complete response (CR) has been associated with improved survival (Palumbo & Cavallo, 2012). However, MM is clinically and pathologically heterogeneous, resulting in variability in both response to treatment and survival. Survival can range from a few months to more than 10 years (Kumar et al., 2012). The MM disease trajectory will vary for each patient; however, relapses are inevitable, and the depth and duration of response following each relapse are generally diminished (Figure 2).
THE PATHOBIOLOGY OF MM
The Malignant Clone and Bone Marrow Microenvironment
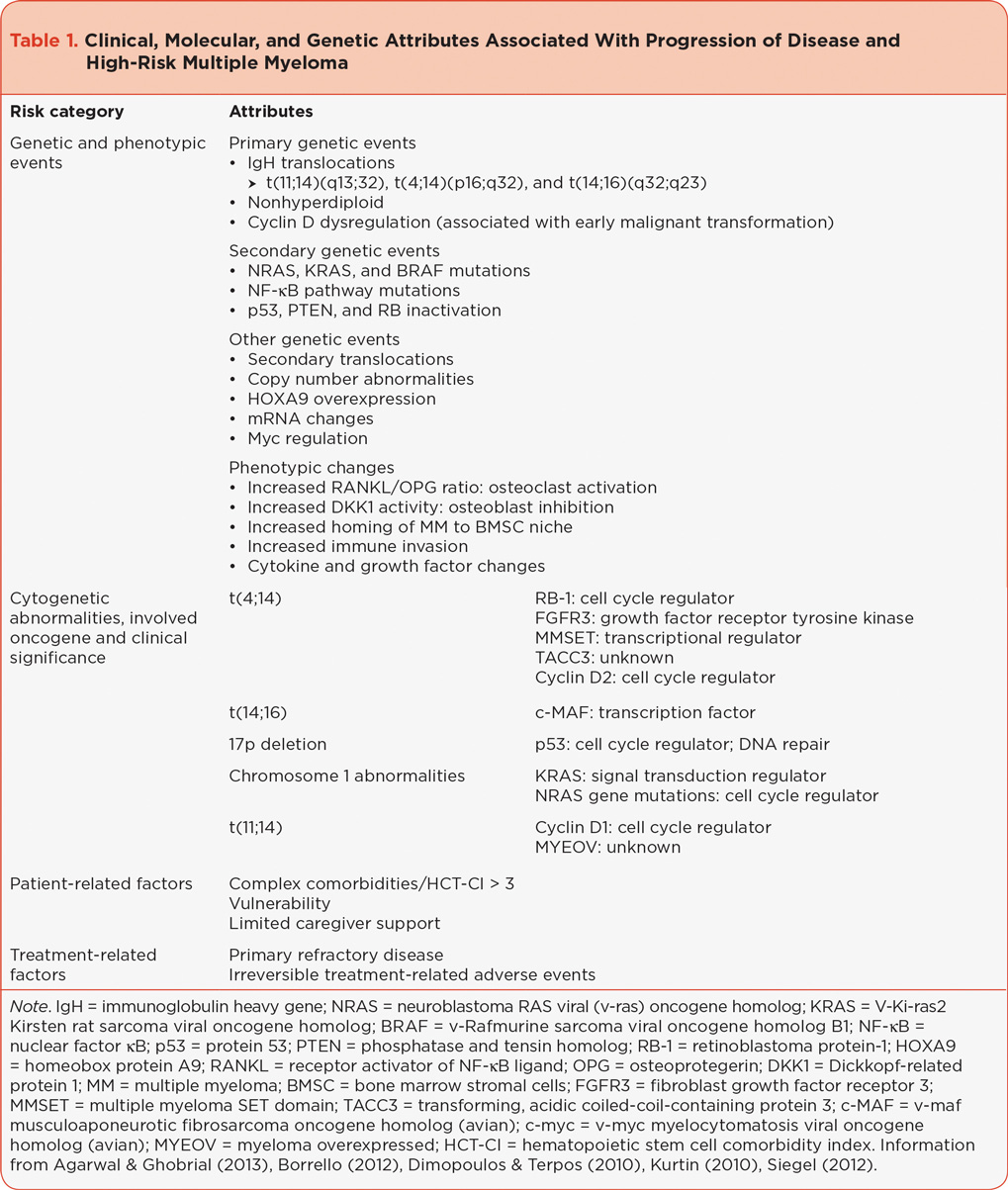
Multiple myeloma is a diverse clonal plasma cell malignancy that results from complex interactions between malignant progenitor cells (mature B lymphocytes), bone marrow stromal cells, and the bone marrow microenvironment. Several factors are thought to play a role in the malignant transformation of plasma cells: chromosome changes, molecular characteristics, and elements that impact the bone marrow microenvironment. Many of these factors are thought to be associated with high-risk MM, with an increased risk of relapse or progression of disease; see Table 1 (Palumbo & Anderson, 2011).
The initiation of myeloma involves genetic events and environmental factors that, when combined with the normal physiologic processes of generating antibodies and interacting with the bone marrow microenvironment, lead to immortalization of a myeloma-propagating clone (Morgan, Walker, & Davies, 2012). The bone marrow microenvironment is structured in compartments or niches comprising hematopoietic and nonhematopoietic cells. The nonhematopoietic cells include stromal cells, adhesion molecules, fibroblasts, osteoclasts, and osteoblasts. B lymphocytes, including normal plasma cells, interact with the stromal cells and the bone marrow microenvironment via various signaling pathways. Deregulation of one or more pathways as a result of genetic and phenotypic changes in the plasma cell clone leads to changes in the bone marrow microenvironment, is implicated in malignant transformation, and contributes to end organ damage (Agarwal & Ghobrial, 2013; Borrello, 2012; Keats et al., 2012). After accrual of sufficient genetic abnormalities, the deregulated plasma cell acquires a clonal advantage, evolves, and expands, contributing to relapse and progression.
A number of molecular abnormalities have been implicated in development of the propagating clone and are associated with high-risk disease (Agarwal & Ghobrial, 2013; Borrello, 2012; Keats et al., 2012; Siegel, 2012). The most common translocations involve the immunoglobulin heavy gene (IgH) locus on chromosome 14 (present in approximately 75% of patients with MM) and result in oncogene dysregulation (Borrello, 2012). Nonhyperdiploid MM, overexpression of cyclin D, and other phenotypic abnormalities—particularly deletion (17), associated with inactivation of p53; deletion (13); and abnormalities of chromosome 1, including 1p22 and 1p32 deletions—are implicated in the pathogenesis of MM and associated with high-risk disease (Borrello, 2012; Kumar et al., 2012). Fluorescence in situ hybridization (FISH) or cytogenetic analysis of t(4;14)(p16;q32), t(14:16)(q32;q23), 17p13 deletions, t(11;14)(q13;q32), chromosome 13 deletion, ploidy category, and chromosome 1 abnormalities are recommended at the initial diagnosis of MM (Fonseca et al., 2009; Kumar, 2010; Siegel, 2012). More recently, gene expression profiling (GEP) has been incorporated into clinical trials. Cytogenetic or molecular responses are not currently incorporated into the response criteria for MM, thus repeat cytogenetics, FISH, or GEP profiles are not routinely used to evaluate response outside of the clinical trial or bone marrow transplant settings (Siegel, 2012).
Adhesion molecules promote homing of the MM cells to the bone marrow stroma and subsequent cytokine and growth factor production (Borrello, 2012). The malignant MM clone is also capable of autocrine production of cytokines. These cytokines promote tumor progression through activation of intracellular pathways, confer a survival advantage to the malignant clone, and contribute to bone involvement and other secondary organ effects common in MM (Siegel, 2012). Interleukin-6 (IL-6) is implicated in the pathogenesis of MM and is thought to confer a proliferative and antiapoptotic advantage that increases treatment resistance and contributes to the pathogenesis of myeloma bone disease and an increased risk of thrombosis (Borrello, 2012; Palumbo & Anderson, 2011).
Tumor necrosis factor–alpha (TNF-á) plays an important role in inflammatory response and bone resorption and is associated with a number of secondary effects that may confer a survival advantage to MM cells, contribute to osteolytic bone disease, and increase the activation of other signaling pathways associated with more aggressive and treatment-resistant disease (Siegel, 2012). Positive cell adhesion-mediated and cytokine-mediated feedback loops support survival of the myeloma clone and can mediate drug resistance. For the patient with RR MM, selection of novel therapies that exploit these highly dysregulated attributes is critical to effective treatment.
Clinical Implications
Inclusion of genetic and phenotypic findings in the original diagnostic evaluation of MM is critical to personalized risk-adapted treatment selection. A number of these attributes are associated with high-risk MM and thought to play a role in decreased survival (Fonseca et al., 2009; Siegel, 2012). Several studies suggest achieving a durable CR is most important in patients with high-risk disease (Durie, 2010; Harousseau, Attal, & Avet-Loiseau, 2009); however, despite achievement of CR, MM remains an incurable disease for the majority of patients. The novel agents bortezomib (Velcade), lenalidomide (Revlimid), carfilzomib (Kyprolis), and pomalidomide (Pomalyst), used in combination with established therapies, including hematopoietic stem cell transplantation (HSCT), are able to neutralize some of these high-risk features and improve outcomes (Richardson et al., 2010). As patients with MM are surviving longer than ever before, patients will be exposed to more MM therapies during the course of their disease.
A percentage of MM patients do not respond to first-line novel agents, and many are not eligible for HSCT, which is the only potentially curative option in MM. Relapse or progression is inevitable for the majority of patients, including those who respond to first-line therapies. Patients who fail first-line proteasome inhibitors or immunomodulatory drugs (IMiDs) have been shown to have poor overall survival, with an average life expectancy of 9 months from the time of becoming refractory to proteasome inhibitors and IMiDs (Kumar et al., 2012).
Responses to RR MM treatment are characteristically short, with a median survival as brief as 6 months (Richardson et al., 2010). Patients with relapsed or relapsed refractory disease represent a heterogeneous population with unique clinical considerations. Effective management of RR MM requires an understanding of the pathobiology of MM, including high-risk features, currently available therapies for all phases of the disease, and the key elements of risk-adapted treatment selection in the RR MM setting, including clinical management of adverse events.
MANAGEMENT OF RELAPSED AND REFRACTORY MULTIPLE MYELOMA TODAY
Relapsed and Refractory Disease
The RR MM population varies based on the type of relapse (early vs. late, or multiple relapses) and the number and types of treatment regimens used. The selection of salvage therapy in this group of patients should be based on careful analysis of individual patient disease characteristics and treatment history (Fonseca et al., 2009). It is essential to understand the definition within the RR MM disease category. The International Myeloma Working Group response criteria and the European Group for Blood and Bone Marrow Transplant include standard definitions for disease progression; see Table 2 (Blade et al., 1998; Durie et al., 2006). Progression of disease is implied in the term “relapsed.” The phrase “relapse from complete remission” is used to describe a patient who develops clinically measurable disease or secondary organ effects after achieving a CR, while “progression” is used to describe a patient who has developed clinically measurable signs of increased disease activity after achieving a partial response (PR) or disease plateau (Anderson et al., 2008; Siegel, 2012). Relapsed and refractory disease is defined as either a lack of response or disease progression on or within 60 days of the last therapy (Anderson et al., 2008). Patients with primary refractory disease have failed to achieve any response to initial MM treatments, often a combination regimen of two or three novel agents. These patients should be encouraged to participate in a clinical trial because they have very high-risk disease and poor prognosis.

Characteristics of the Relapsed/Refractory Patient
The traditional measures of eligibility for clinical trials have relied on estimates by clinicians of functional and/or performance status (PS), considering activities of daily living and independent activities of daily living (Oken et al., 1982; Schag, Heinrich, & Ganz, 1984). Similar approaches are used in treatment of patients outside of the clinical trial setting. Performance status information is garnered from both assessment of the patient as well as discussion with the patient and family.
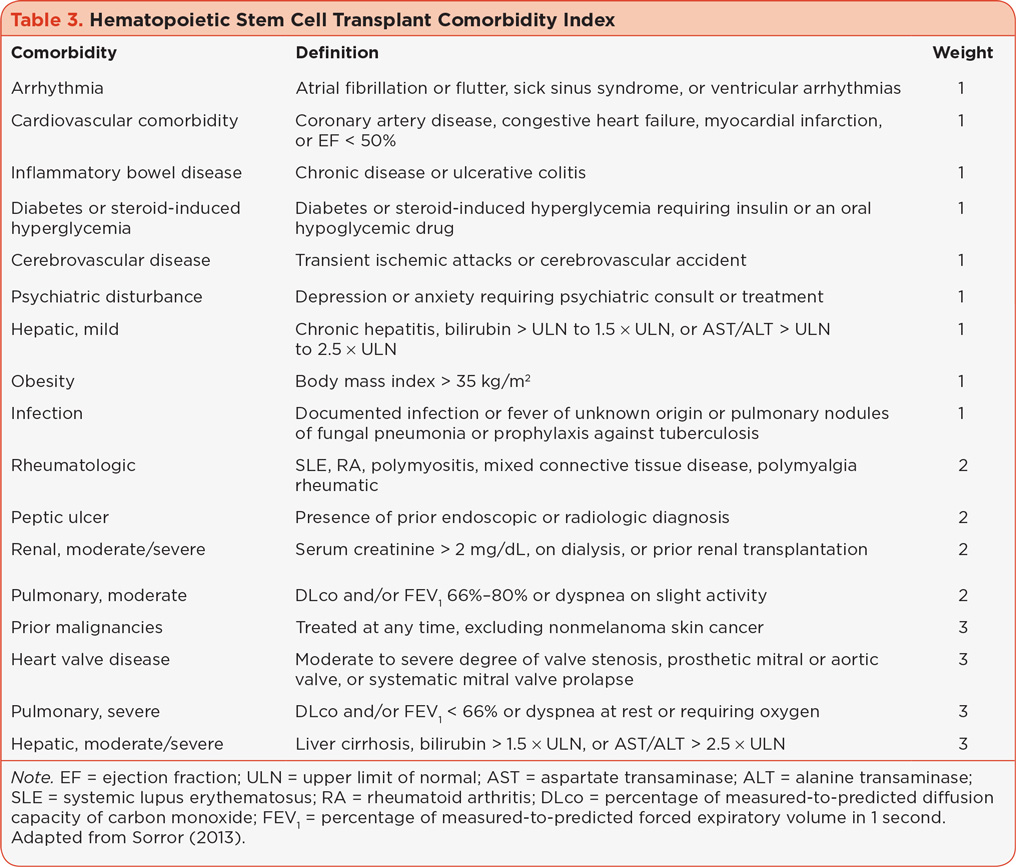
Frailty and vulnerability has been found to correlate with unfavorable outcome. Palumbo and colleagues (Palumbo et al., 2011) introduced the concept of vulnerability, which incorporates evaluation of PS, frailty, and comorbidities. The evaluation of vulnerability is considered critical to the risk-adapted treatment selection for MM patients being considered for HSCT. The hematopoietic stem cell comorbidity index (HCT-CI) attributes numerical scores to 17 different categories of organ dysfunction associated with unfavorable outcomes in the HSCT population; see Table 3 (Sorror, 2013). A HCT-CI score greater than 3 is associated with inferior nonrelapsed mortality in the HSCT population. However, patients may have a better PS because their disease is not as aggressive, which may result in selection bias for HSCT. Multiple myeloma remains the most common diagnosis referred for autologous HSCT (auto-HSCT), and many patients with RR MM have undergone at least one auto-HSCT. Thus, a similar approach to selecting treatment in the RR MM population should incorporate assessment of comorbidities with consideration of the available salvage therapies and their specific adverse event profiles.
Treatment Selection for RR MM
Management of RR MM requires careful evaluation of each individual patient to include the characteristics of disease at the time of original diagnosis, changes in disease characteristics over time, treatment history and response, and individual patient characteristics (Table 4). Treatment in the RR MM setting is considered to be salvage therapy; however, patients who have received limited prior therapies may benefit from a number of available novel agents or combinations that are used in the first-line setting (Eshaghian & Berenson, 2012; van de Donk et al., 2011). The goal of salvage therapy in the RR MM population is to achieve disease control with acceptable toxicity and an acceptable or improved quality of life. Treatment should continue until disease progression or unacceptable toxicity and with consideration of the patient’s wishes. Care should be used in selecting agents based on transplant eligibility and residual toxicities. The depth and duration of response to prior therapies should be evaluated. Patients who have failed or are intolerant to first-line novel agents—specifically lenalidomide or bortezomib—should be considered for the newly approved novel agents carfilzomib and pomalidomide (see Table 5;NCCN, 2013).
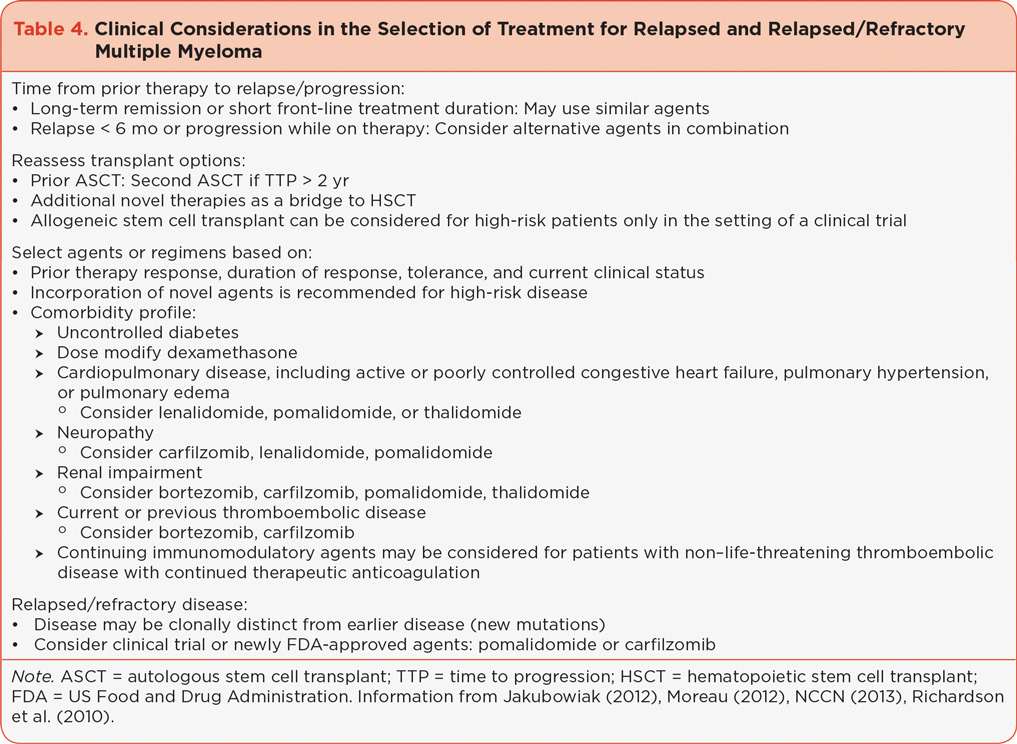

Given the number of emerging treatment options, including combinations using novel agents currently approved as single agents, avoiding irreversible toxicities that may prevent benefit from these treatments is imperative. Supportive care, including bisphosphonate therapy for bone health, infection prophylaxis, nutritional support, and maintenance of physical activity, should continue for all patients with MM (Snowden et al., 2011).
CONCLUSIONS AND FUTURE CHALLENGES
The integration of novel agents into the treatment of MM offers the possibility of long-term survival and quality of life (Kumar et al., 2008; Jordan et al., 2013). Patients with RR MM present a unique challenge requiring careful consideration of specific disease, treatment, and individual attributes (Jakubowiak, 2012; Moreau, 2012; Palumbo et al., 2011; Palumbo & Anderson, 2011; Siegel, 2012; van de Donk et al., 2011). Maintaining familiarity with the patient over the course of their disease is optimal but not always possible.
Ongoing evaluation of response requires working knowledge of the pathobiology of MM, clinical findings, current criteria for evaluation of response, and secondary options for treatment. Proteasome inhibitors and IMiDs are the backbone of current standard therapies for the treatment of MM. Recent trials and next-generation agents, including carfilzomib and pomalidomide, are particularly important for patients with relapsed and refractory disease. The advanced practice provider (APP) in oncology plays an integral role in managing patients with MM over the course of their disease, monitoring response to treatment, and identifying progression or relapse. Familiarity with emerging therapies will assist the APP in the early identification and treatment of common adverse events and improve patient care.
ACKNOWLEDGMENT
The author would like to thank Melissa Kirk, PhD (Fishawack Communications), for editorial assistance, which was supported by Onyx Pharmaceuticals, Inc.
DISCLOSURE
Ms. Kurtin has acted as a consultant for Onyx, Celgene, and Millennium.
REFERENCES
Agarwal, A., & Ghobrial, I. M. (2013). Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: A review of the current understanding of epidemiology, biology, risk stratification, and management of myeloma precursor disease. Clinical Cancer Research, 19(5), 985–994. http://dx.doi.org/10.1158/1078-0432.ccr-12-2922
American Cancer Society. (2013). Cancer Facts & Figures 2013. Atlanta: American Cancer Society.
Anderson, K. C., Kyle, R. A., Rajkumar, S. V., Stewart, A. K., Weber, D., & Richardson, P. (2008). Clinically relevant end points and new drug approvals for myeloma. Leukemia, 22(2), 231–239. http://dx.doi.org/10.1038/sj.leu.2405016
Blade, J., Samson, D., Reece, D., Apperley, J., Bjorkstrand, B., Gahrton, G.,...Vesole, D. (1998). Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. British Journal of Haematology, 102(5), 1115–1123.
Borrello, I. (2012). Can we change the disease biology of multiple myeloma? Leukemia Research, 36, S3–S12. http://dx.doi.org/10.1016/s0145-2126(12)70003-6
Celgene Corporation. (2013). Pomalyst (pomalidomide) package insert. Retrieved from http://www.pomalyst.com/docs/prescribing_information.pdf
Dimopoulos, M. A., & Terpos, E. (2010). Multiple myeloma. Annals of Oncology, 21(suppl 7), vii143–vii150. http://dx.doi.org/10.1093/annonc/mdq370
Durie, B. G. (2010). Role of new treatment approaches in defining treatment goals in multiple myeloma—The ultimate goal is extended survival. Cancer Treatment Review, 36(suppl 2), S18–S23. http://dx.doi.org/10.1016/s0305-7372(10)70008-6
Durie, B. G., Harousseau, J. L., Miguel, J. S., Blade, J., Barlogie, B., Anderson, K.,...Rajkumar, S. V. (2006). International uniform response criteria for multiple myeloma. Leukemia, 20(9), 1467–1473. http://dx.doi.org/10.1038/sj.leu.2404284
Durie, B. G., Kyle, R. A., Belch, A., Bensinger, W., Blade, J., Boccadoro, M.,...Van Ness, B. (2003). Myeloma management guidelines: A consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematology Journal, 4(6), 379–398. http://dx.doi.org/10.1038/sj.thj.6200312
Eshaghian, S., & Berenson, J. R. (2012). Multiple myeloma: Improved outcomes with new therapeutic approaches. Current Opinion in Supportive and Palliative Care, 6(3), 330–336. http://dx.doi.org/10.1097/SPC.0b013e3283565c56
Fonseca, R., Bergsagel, P. L., Drach, J., Shaughnessy, J., Gutierrez, N., Stewart, A. K.,...Avet-Loiseau, H. (2009). International Myeloma Working Group molecular classification of multiple myeloma: Spotlight review. Leukemia, 23(12), 2210–2221. http://dx.doi.org/10.1038/leu.2009.174
Harousseau, J. L., Attal, M., & Avet-Loiseau, H. (2009). The role of complete response in multiple myeloma. Blood, 114(15), 3139–3146. http://dx.doi.org/10.1182/blood-2009-03-201053
Jakubowiak, A. (2012). Management strategies for relapsed/refractory multiple myeloma: Current clinical perspectives. Seminars in Hematology, 49(suppl 1), S16–S32. http://dx.doi.org/10.1053/j.seminhematol.2012.05.003
Jordan, K., Proskorovsky, I., Lewis, P., Ishak, J., Payne, K., Lordan, N.,…Davies, F. E. (2013). Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: Results of a European, multicenter cohort study. Supportive Care in Cancer [E-pub ahead of print].
Keats, J. J., Chesi, M., Egan, J. B., Garbitt, V. M., Palmer, S. E., Braggio, E.,...Bergsagel, P. L. (2012). Clonal competition with alternating dominance in multiple myeloma. Blood, 120(5), 1067–1076. http://dx.doi.org/10.1182/blood-2012-01-405985
Kuehl, W. M., & Bergsagel, P. L. (2012). Molecular pathogenesis of multiple myeloma and its premalignant precursor. Journal of Clinical Investigation, 122(10), 3456–3463. http://dx.doi.org/10.1172/JCI61188
Kumar, S. (2010). Multiple myeloma—Current issues and controversies. Cancer Treatment Reviews, 369(suppl 2), S3–S11. http://dx.doi.org/10.1016/S0305-7372(10)70006-2
Kumar, S. K., Lee, J. H., Lahuerta, J. J., Morgan, G., Richardson, P. G., Crowley, J.,...Durie, B. G. (2012). Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter International Myeloma Working Group study. Leukemia, 26(1), 149–157. http://dx.doi.org/10.1038/leu.2011.196
Kumar, S. K., Rajkumar, S. V., Dispenzieri, A., Lacy, M. Q., Hayman, S. R., Buadi, F. K.,…Gertz, M. A. (2008). Improved survival in multiple myeloma and the impact of novel therapies. Blood, 111(5), 2516–2520. http://dx.doi.org/10.1182/blood-2007-10-116129
Kurtin, S. (2010). Laboratory measures for the diagnosis, clinical management, and evaluation of treatment response in multiple myeloma. Journal of the Advanced Practitioner in Oncology, 1, 197–206.
Kurtin, S. (2012). Myeloid toxicity of cancer treatment. Journal of the Advanced Practitioner in Oncology, 3, 209–224.
Moreau, P. (2012). The future of therapy for relapsed/refractory multiple myeloma: Emerging agents and novel treatment strategies. Seminars in Hematology, 49(suppl 1), S33–S46. http://dx.doi.org/10.1053/j.seminhematol.2012.05.004
Morgan, G. J., Walker, B. A., & Davies, F. E. (2012). The genetic architecture of multiple myeloma. Nature Reviews Cancer, 12(5), 335–348. http://dx.doi.org/10.1038/nrc3257
National Cancer Institute. (2013). Surveillance Epidemiology and End Results (SEER) Stat Fact Sheets: Myeloma. Retrieved from http://seer.cancer.gov/statfacts/html/mulmy.html#incidence-mortality
National Comprehensive Cancer Network. (2013). NCCN Clinical Practice Guidelines in Oncology: Multiple myeloma, version 2.2013.
Oken, M. M., Creech, R. H., Tormey, D. C., Horton, J., Davis, T. E., McFadden, E. T., & Carbone, P. P. (1982). Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology, 5(6), 649–655.
Onyx Pharmaceuticals. (2012). Kyprolis (Carfilzomib) package insert. Retrieved from http://www.kyprolis.com/prescribing-information
Onyx Pharmaceuticals. (2013). Pomalyst (pomalidomide) package insert. Retrieved from http://www.pomalyst.com/docs/prescribing_information.pdf
Palumbo, A., & Anderson, K. (2011). Multiple myeloma. New England Journal of Medicine, 364(11), 1046–1060. http://dx.doi.org/10.1056/NEJMra1011442
Palumbo, A., Bringhen, S., Ludwig, H., Dimopoulos, M. A., Blade, J., Mateos, M. V.,...Sonneveld, P. (2011). Personalized therapy in multiple myeloma according to patient age and vulnerability: A report of the European Myeloma Network (EMN). Blood, 118(17), 4519–4529. http://dx.doi.org/10.1182/blood-2011-06-358812
Palumbo, A., & Cavallo, F. (2012). Have drug combinations supplanted stem cell transplantation in myeloma? American Society of Hematology Education Program, 2012, 335–341. http://dx.doi.org/10.1182/asheducation-2012.1.335
Perrotta, C., Kleefeld, S., Staines, A., Tewari, P., De Roos, A. J., Baris, D.,...Cocco, P. (2013). Multiple myeloma and occupation: A pooled analysis by the International Multiple Myeloma Consortium. Cancer Epidemiology, 37(3), 300–305. http://dx.doi.org/10.1016/j.canep.2013.01.008
Pingali, S. R., Haddad, R. Y., & Saad, A. (2012). Current concepts of clinical management of multiple myeloma. Disease-a-Month, 58(4), 195–207. http://dx.doi.org/10.1016/j.disamonth.2012.01.006
Raab, M. S., Podar, K., Breitkreutz, I., Richardson, P. G., & Anderson, K. C. (2009). Multiple myeloma. Lancet, 374(9686), 324–339. http://dx.doi.org/10.1016/S0140-6736(09)60221-X
Richardson, P. G., Laubach, J., Mitsiades, C., Schlossman, R. L., Doss, D., Colson, K.,...Anderson, K. (2010). Tailoring treatment for multiple myeloma patients with relapsed and refractory disease. Oncology (Williston Park), 24(3 suppl 2), 22–29.
Schag, C. C., Heinrich, R. L., & Ganz, P. A. (1984). Karnofsky performance status revisited: Reliability, validity, and guidelines. Journal of Clinical Oncology, 2(3), 187–193.
Siegel, D. S. (2012). Relapsed/refractory multiple myeloma: Defining refractory disease and identifying strategies to overcome resistance. Seminars in Hematology, 49(suppl 1), S3–S15. http://dx.doi.org/10.1053/j.seminhematol.2012.05.005
Snowden, J. A., Ahmedzai, S. H., Ashcroft, J., D’Sa, S., Littlewood, T., Low, E.,…Bird, J. M. (2011). Guidelines for supportive care in multiple myeloma 2011. British Journal of Haematology, 154(1), 76–103. http://dx.doi/org/10.1111/j.1365-2141.2011.08574.x
Sorror, M. L. (2013). How I assess comorbidities before hematopoietic cell transplantation. Blood, 121(15), 2854–2863. http://dx.doi.org/10.1182/blood-2012-09-455063
Vacca, A., & Ribatti, D. (2006). Bone marrow angiogenesis in multiple myeloma. Leukemia, 20(2), 193–199. http://dx.doi.org/10.1038/sj.leu.2404067
van de Donk, N. W., Lokhorst, H. M., Dimopoulos, M., Cavo, M., Morgan, G., Einsele, H.,...Palumbo, A. (2011). Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treatment Reviews, 37(4), 266–283. http://dx.doi.org/10.1016/j.ctrv.2010.08.008