Meeting Abstract
JL413. Management of Gastrointestinal Adverse Events (AEs) Associated With Nivolumab and Ipilimumab Combination Therapy in Solid Tumors
Lisa Kottschade, APRN, MSN, CNP, Mayo Clinic, Rochester, MN, Marianne Davies, DNP, ACNP, AOCNP®, Yale University School of Medicine, New Haven, CT, Krista Rubin, MS, FNP-BC, Massachusetts General Hospital, Boston, MA, Kathleen Madden, NP, MSN, FNP-BC, AOCNP®, APHN, New York University, New York, NY, Nathan Dahl, PharmD, RPh, Mayo Clinic, Rochester, MN, Laura Brennan, NP, AOCNP®, UC Davis Medical Center, Sacramento, CA, Dana Walker, MD, MSCE, Bristol-Myers Squibb, Princeton, NJ, Xuemei Li, MD, Bristol-Myers Squibb, Princeton, NJ, Paul Gagnier, PhD, MD, Bristol-Myers Squibb, Princeton, NJ, and Laura S. Wood, RN, MSN, OCN®, Cleveland Clinic, Cleveland, OH
 ABSTRACT
ABSTRACT
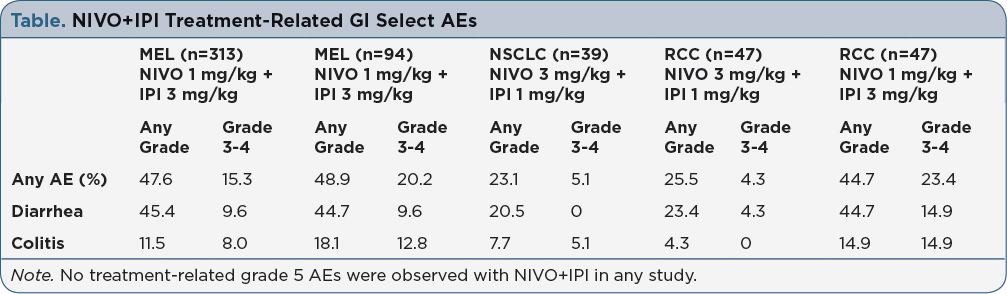
Background: Nivolumab (NIVO) and ipilimumab (IPI) combination therapy is approved for the first-line treatment of advanced melanoma (MEL), and recent phase 1 studies have shown promising efficacy in non-small cell lung cancer (NSCLC) and metastatic renal cell carcinoma (RCC). NIVO+IPI is associated with a higher frequency of treatment-related AEs compared to monotherapy, with select AEs (ie, those with an immune-related etiology) in the skin and gastrointestinal (GI) tract being the most common. Here, we review the incidence of GI select AEs in patients (pts) treated with NIVO+IPI and present practical guidance regarding their management. Methods: Safety data were included from phase 2 (CheckMate 069) and phase 3 (CheckMate 067) studies for MEL, and phase 1 studies for NSCLC (CheckMate 012) and RCC (CheckMate 016). Data are reported for NIVO 1 mg/kg + IPI 3 mg/kg Q3W x 4 for MEL, NIVO 3 mg/kg Q2W + IPI 1 mg/kg Q6W for NSCLC, and NIVO 3 + IPI 1 or NIVO 1 + IPI 3 mg/kg Q3W x 4 for RCC, followed by NIVO Q2W. Pts were treated until disease progression, unacceptable toxicity, or withdrawal of consent. Results: In MEL pts treated with NIVO+IPI, ~48% experienced any grade GI select AEs; 15-20% experienced grade 3-4 AEs, with the most common being diarrhea and colitis (Table). Any grade GI select AEs occurred in 23% of pts with NSCLC, in 26% of pts with RCC who received NIVO 3 + IPI 1 mg/kg, and in 45% of pts with RCC who received NIVO 1 + IPI 3 mg/kg. To effectively manage GI AEs, pts need to report all GI symptoms (e.g., increased stool frequency, loose consistency, abdominal cramping, and bloody diarrhea) promptly to their oncology healthcare provider. Pts with diarrhea should undergo stool testing to rule out infectious causes. GI AEs can progress rapidly, and thus healthcare providers should initiate treatment at the first sign of symptoms (corticosteroids for pts with persistent grade ≥2 AEs) even before stool testing results are available, in order to prevent serious complications of intestinal inflammation such as bowel perforation. Implications: Across solid tumor types, GI select AEs are among the most commonly reported side effects in pts who receive NIVO+IPI therapy. With early recognition and intervention, serious complications from GI select AEs may be minimized using established management guidelines involving immune modulating medications. Pt education and frequent follow-up are also key to minimizing serious complications from these AEs.
For access to the full length article, please
sign in.