Grand Rounds
Multidisciplinary Management of the Patient With Metastatic Colorectal Adenocarcinoma
Steve A. Malangone, MSN, NP-C, Hitendra Patel, MD, Sandra E. Kurtin, RN, MS, AOCN®, ANP-C, Hina Arif Tiwari, MD, and Emad Elquza, MD
University of Arizona Cancer Center, Tucson, Arizona
Authors’ disclosures of potential conflicts of interest are found at the end of this article.
Steve A. Malangone, MSN, NP-C, University of Arizona Cancer Center, 3838 North Campbell Avenue, Tucson, AZ 85719. E-mail: steve.malangone@uahealth.com
J Adv Pract Oncol 2015;6:144–152 |
DOI: 10.6004/jadpro.2015.6.2.6 |
© 2015 Harborside Press®
 ABSTRACT
ABSTRACT
View Continuing Education Information here.
Case Study
A 60-year-old man initially presented with rectal bleeding in March 2006. He underwent a colonoscopy, which revealed a rectosigmoid mass, and the biopsy specimen confirmed adenocarcinoma.
In May 2006, the patient underwent planned low anterior resection. During the procedure, he was found to have a metastatic lesion in the left hepatic lobe. Hepatobiliary surgery was consulted, and a left lateral liver resection was performed at the time of the initial operation. Complete surgical pathology revealed T3, N2, M1, with 11 of 28 lymph nodes positive for disease.
The patient then went on to receive postoperative treatment with fluorouracil (5-FU), leucovorin, and oxaliplatin (FOLFOX) and bevacizumab (Avastin). His treatment was interrupted due to sudden cardiac arrest requiring resuscitation measures for ventricular fibrillation. He had an implanted defibrillator placed and resumed treatment with 5-FU/oxaliplatin and bevacizumab chemotherapy. He was then changed to 5-FU and weekly oxaliplatin with concurrent radiotherapy. This treatment was completed in October 2006. Afterward, the patient completed an additional 4 cycles of FOLFOX/bevacizumab, with the last dose given in December 2006.
The patient remained on surveillance without evidence of tumor recurrence for more than 3.5 years, until a September 2010 CT scan revealed a segment 7 hepatic lesion. He was treated with 3 cycles of 5-FU/irinotecan (FOLFIRI).
His case was discussed in the multidisciplinary colorectal tumor conference. He was not deemed a candidate for further hepatic resection due to the location of the lesion and prior extensive resection. Various approaches to therapy were discussed, and he was taken for exploratory laparotomy with intraoperative ultrasonography of the liver and radiofrequency ablation of a single lesion in segment 7. He was treated with an additional 2 months of FOLFIRI through April 2011.
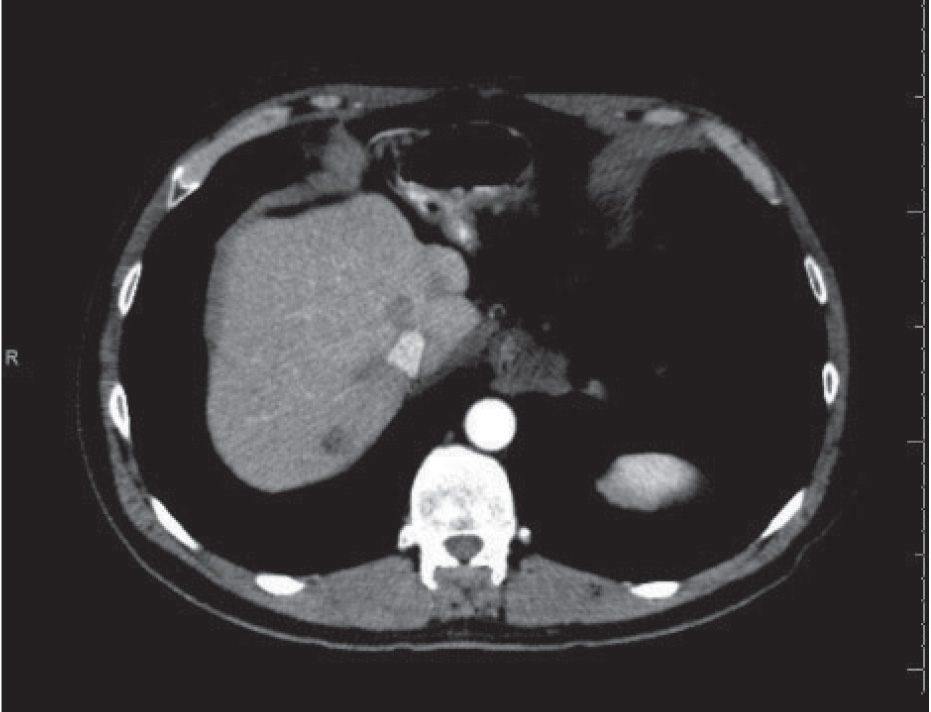
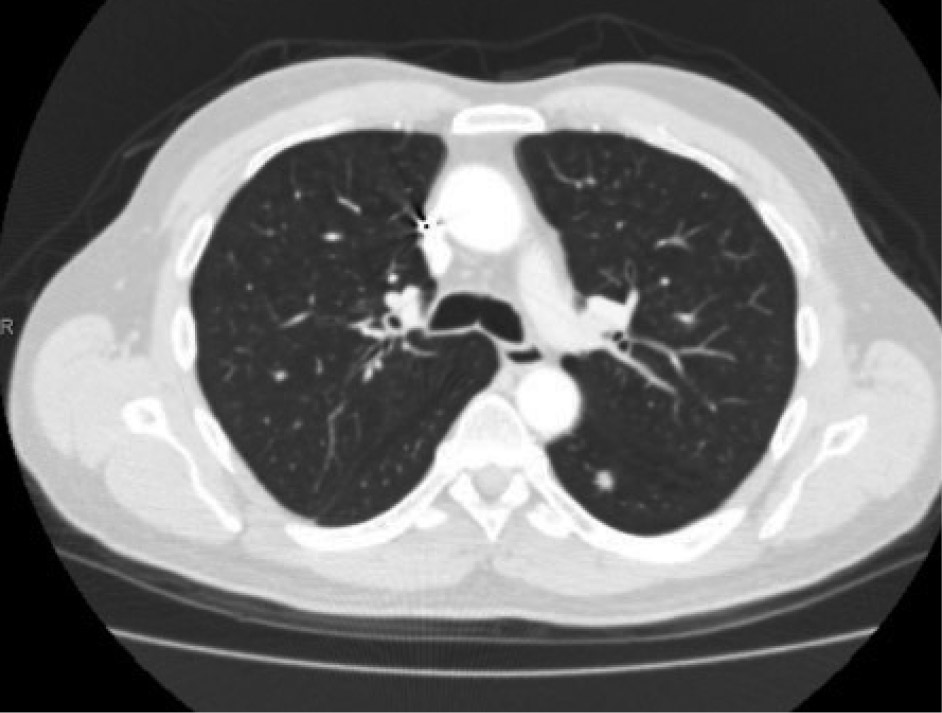
In September 2011, a CT scan of the chest, abdomen, and pelvis revealed enlargement of a previously demonstrated segment 7 hepatic lesion and a 7-mm left lower-lobe pulmonary nodule (Figures 1 and 2). CT-guided biopsy of the lung lesion confirmed metastatic colorectal cancer. At that time, KRAS testing was requested, which revealed no KRAS mutation. The patient was treated with capecitabine/oxaliplatin/cetuximab (Erbitux) for 7 cycles. In August 2012, a follow-up PET/CT scan revealed a complete metabolic response (Figure 3). He was then transitioned to single-agent weekly cetuximab.
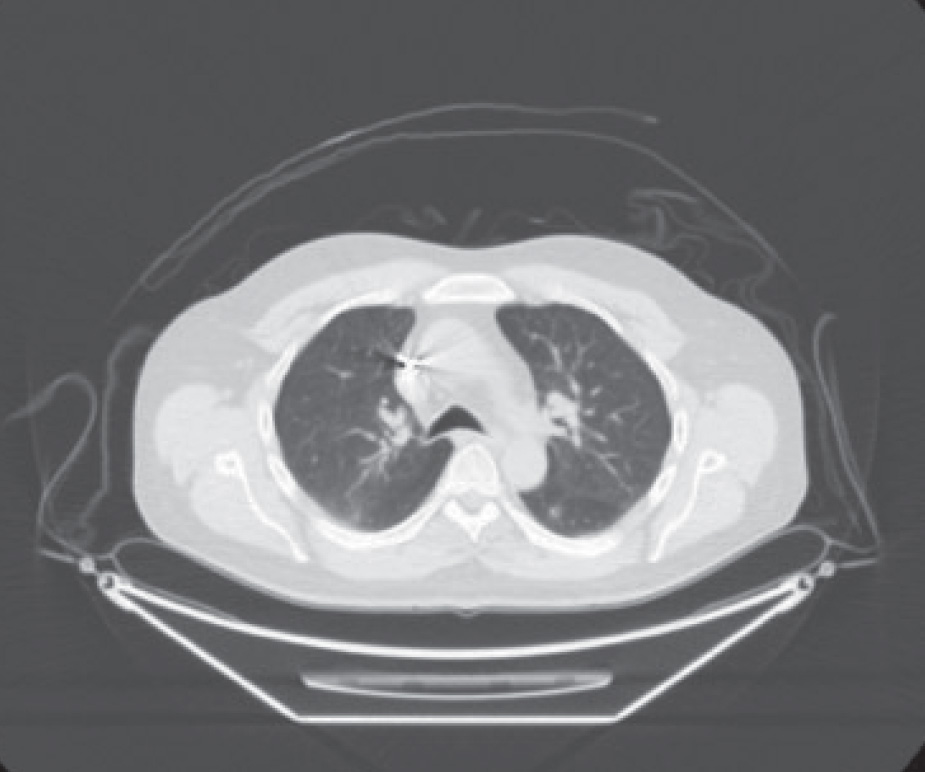

In April 2013, a PET-CT scan revealed an increase in metabolic activity and size of the left lower-lobe lung lesion (Figure 4). His case was discussed at the colorectal Multidisciplinary Tumor Conference; based on this discussion, he was referred to radiation oncology for stereotactic body radiation therapy (SBRT) of the pulmonary nodule, which was completed at the end of May 2013. A PET/CT scan obtained in September 2013 revealed evidence of a good response to the left pulmonary lesion and no evidence of new or recurrent disease.
In December 2013, a subsequent PET/CT scan revealed a single lesion in the liver at the dome and no disease elsewhere. This lesion was present in 2012 and disappeared with treatment. Again, this case was discussed at the colorectal multidisciplinary tumor conference, and the patient was referred for radiofrequency ablation to this lesion, which was completed in February 2014.
A follow-up PET-CT scan in mid-March 2014 revealed a good response to therapy, with no evidence of viable tumor. Unfortunately, PET/CT completed in late June 2014 revealed two new fluorodeoxyglucose (FDG)-avid lesions in the liver in segments 6 and 7. Carcinoembryonic antigen (CEA) also was elevated (6.3 ng/mL, compared with 4.7 ng/mL in March 2014).
The case was once again discussed at the colorectal Multidisciplinary Tumor Conference. The segment 7 lesion was identified as recurrence at the periphery of a previously treated lesion. The patient was referred back to interventional radiology for consideration of additional liver-directed therapy. In addition, given the rising CEA level and the clinical picture suggestive of systemic tumor progression, the patient was restarted on systemic chemotherapy with irinotecan/panitumumab (Vectibix).
For access to the full length article, please
sign in.