Grand Rounds
Carboplatin Hypersensitivity in Recurrent Ovarian Cancer
Paula J. Anastasia, RN, MN, AOCN®
From Cedars-Sinai Medical Center, Los Angeles, California
The author has no conflicts of interest to disclose.
Correspondence to: Paula J. Anastasia, RN, MN, AOCN®, 8700 Beverly Boulevard, Suite 290W, Los Angeles, CA 90048.
E-mail: paula.anastasia@cshs.org
J Adv Pract Oncol 2011;2:177–184 |
DOI: 10.6004/jadpro.2011.2.3.4 |
© 2011 Harborside Press®
 ABSTRACT
ABSTRACT
Case Study
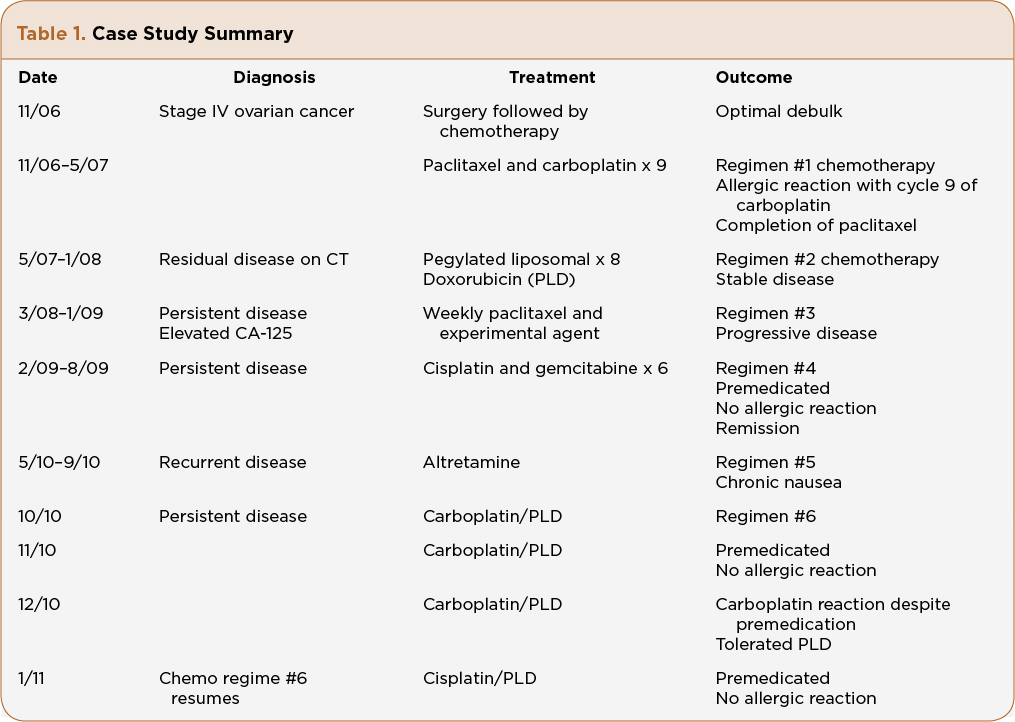
Mrs. A.Z. is a 61-year-old Caucasian woman who was diagnosed with stage IV (pleural effusion cytology positive), grade 3, papillary serous adenocarcinoma of the ovary at age 56. She underwent standard of care procedures with an optimally debulked surgery, followed by IV paclitaxel and carboplatin every 3 weeks. Her CA-125 was elevated prior to surgery and had a slower than expected decline to normal values (< 35 U/mL) even after 6 cycles of chemotherapy. Her medical oncologist made the decision to give three more cycles of chemotherapy and reevaluate with a computerized axial tomography (CT) scan. During the ninth cycle of chemotherapy, 1 hour after completion of paclitaxel and 15 minutes into her last carboplatin infusion, Mrs. A.Z. reported erythema of the palms, throat tightness, and abdominal pain. The carboplatin infusion was stopped. The patient was given additional dexamethasone and an antihistamine. The symptoms resolved immediately and the decision was made to discontinue further carboplatin. Her medical oncologist recommended 8 monthly cycles of PLD for ”mild stranding around the bowel,” which was seen on CT scan. Her CA-125 remained in the upper limits of normal. Mrs. A.Z. tolerated PLD for 8 months without significant adverse events.
Mrs. A.Z. presented to our facility for a second opinion, 2 months after completing her eighth PLD. Her CA-125 continued to increase and her medical oncologist told her she had limited options because she was platinum resistant and platinum allergic. After a thorough patient history, physical, and review of her previous scans with the radiologist, treatment options were discussed, including using a platinum-based regimen since her last platinum was 9 months prior. Mrs. A.Z. verbalized fear and apprehension regarding receiving a platinum-based regime, despite the rationale of a desensitization regime. She chose to transfer her care to our practice and agreed to enroll in a clinical trial consisting of weekly paclitaxel and a targeted agent. She remained on the clinical trial for 10 months, and was taken off due to rising CA-125, even though the CT scan remained stable. Again, discussion regarding different treatment options included a nonplatinum single-agent chemotherapy or a combination platinum-based treatment. She agreed to be treated with a carboplatin substitute and her next treatment would consist of cisplatin 35 mg/m2 and gemcitabine 800 mg/m2 IV on days 1 and 8 in a 21-day cycle.
Mrs. A.Z. was instructed to premedicate with oral dexamethasone 20 mg, 12 hours and 6 hours prior to infusion. She would receive additional IV dexamethasone 20 mg prior to cisplatin infusion, in addition to oral loratidine 10 mg (she stated diphenhydramine causes “restlessness”) and cimetidine 300 mg IV. She also received antiemetic coverage with a long-acting 5-HT3 antagonist and NK1 receptor antagonist. A 4-mg oral dexamethasone taper was given on days 2 and 3 following chemotherapy. The infusion nurse was informed about Mrs. A.Z.’s prior allergic reaction that occurred more than 2 years ago in another facility. No skin testing was done on Mrs. A.Z. prior to her cisplatin. Skin testing is per physician discretion and not a standard protocol at our facility. An emergency kit was available at Mrs. A.Z.’s bedside, including oxygen. There was no HSR after six complete cycles of cisplatin and gemcitabine using this aggressive premedication regime. Mrs. A.Z.’s follow-up PET/CT showed no evidence of disease.
Nine months later, after another relapse of her disease, Mrs. A.Z. started oral chemotherapy with altretamine (Hexalen). She tolerated it for 5 months, then requested a change due to chronic nausea. Once again the decision to reintroduce carboplatin was discussed, this time in combination with PLD. Data from Pujade-Lauraine et al. (2010) reported less HSR with carboplatin when combined with PLD, and Mrs. A.Z. did tolerate PLD in the early part of her diagnosis, which was the reason to repeat this agent. Realizing Mrs. A.Z. was at risk for another carboplatin HSR, she was premedicated with the same regime used with her prior cisplatin and gemcitabine. The carboplatin dose was decreased to AUC 5 instead of 6 when given in combination of PLD. It was also infused over 3 hours instead of 1 hour, which is consistent with rapid desensitization schedules (O’Cearbhaill et al., 2010).
Mrs. A.Z. received two cycles of PLD and carboplatin without incident. Her CA-125 had decreased after both cycles, which validated response to treatment. On cycle 3, Mrs. A.Z. received carboplatin prior to PLD, and within 15 minutes of the infusion she reported palmar itching and burning, throat tightening, and abdominal pain. The infusion was immediately stopped. Oxygen saturation was 92% then increased to 95%. The physician was called and 50 mg of diphenhydramine was administered, despite the patient’s history of restlessness with drug. Her vital signs stabilized and she reported feeling better immediately. After 30 minutes of observation, it was decided to hold carboplatin, despite stabilization, and proceed with the planned PLD. She tolerated the PLD without incidence.
One month later, Mrs. A.Z. returned for her next cycle of chemotherapy. She was switched to PLD 30 mg/m2 and cisplatin 50 mg/m2 every 4 weeks (Table 1). She was instructed to premedicate the day before with oral dexamethasome 20 mg, 12 hours and 6 hours prior to infusion. On the day of infusion she received IV dexamethasone 20 mg, IV ranitidine 50 mg, and oral loratadine 10 mg. The PLD was administered prior to cisplatin. An additional dose of 8-mg dexamethasone IV was infused prior to cisplatin. Mrs. A.Z. tolerated her platinum-based chemotherapy without incident. She was instructed to begin an oral dexamethasone 4-mg taper on days 2 and 3, along with follow-up with her primary care physician to monitor glucose levels. In addition, she was instructed to call with any allergy-like symptoms or unresolved nausea, vomiting, and fever.
For access to the full length article, please
sign in.