The Side Effect Profile of Carfilzomib: From Clinical Trials to Clinical Practice
From John Theurer Cancer Center at Hackensack University Medical Center, Hackensack, New Jersey, and MD Anderson Cancer Center, Houston, Texas
Ms. McBride has received payment as a member of the Onyx Speakers Bureau and reimbursement for travel, accommodations, and meeting expenses from L&M Healthcare Communications. Ms. Samuel has received an honorarium as a member of the Carfilzomib Advisory Board.
Correspondence to: Christine O. Samuel, RN, BSN, OCN®, CCRP, Lymphoma/Myeloma Center, MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030. E-mail: cosamuel@mdanderson.org
J Adv Pract Oncol 2013;4(Suppl 1):22–30 | © 2013 Harborside Press®


ABSTRACT
Treatment of patients with relapsed and/or refractory multiple myeloma (RR MM) is challenging due to the heterogeneity of the disease, patient comorbidities, and side effects of individual treatments. Carfilzomib, a selective proteasome inhibitor that has shown safety and efficacy as a single agent in phase I and II trials, was recently approved by the US Food and Drug Administration for RR MM. The aim of this review is to summarize adverse events (AEs) in patients with MM treated with single-agent carfilzomib in phase II clinical studies and to provide insight into the prevention and management of these AEs from the perspective of the advanced practitioner (AP). Data from a cross-trial safety analysis describing the safety and tolerability of single-agent carfilzomib in 526 patients with RR MM who received carfilzomib in four phase II trials are summarized. Additional information is included based on AP clinical practice experience gained in the clinical trial setting. Guidelines are suggested to assist with patient management prior to carfilzomib treatment, including hydration and prophylactic medication, as well as the management of hematologic AEs, fatigue, and dyspnea during treatment. Infrequent but potentially serious AEs, including cardiac and renal complications, are also reviewed. Adverse events in varying frequencies occur in response to carfilzomib, but they can be managed with prophylaxis and routine care. Utilizing the clinical practice experience with carfilzomib presented here should help to maximize the efficacy of carfilzomib while minimizing AEs and potential discomfort in patients with late-stage MM.
ARTICLE
Multiple myeloma (MM) is a hematologic cancer that is expected to result in more than 10,700 deaths in the United States in 2013 (American Cancer Society, 2013). Multiple myeloma mainly manifests as hypercalcemia, renal insufficiency, anemia, and bone lesions, mnemonically referred to as CRAB (Raab, Podar, Breitkreutz, Richardson, & Anderson, 2009). Patients can range from asymptomatic to severely disabled, depending on where they are in the course of their disease (Pingali et al., 2012). Although new treatments have improved outcomes for patients with MM, the disease remains incurable and is associated with limited survival (Laubach et al., 2011; Ludwig et al., 2011).
Along with the clinical manifestations of the disease, it is important to be aware of the common side effects of MM treatments; these can include myelosuppression, infection, peripheral neuropathy (PN), thromboembolic complications, and untoward cardiac side effects (Pingali, Haddad, & Saad, 2012). Treatment of patients with relapsed and/or refractory (RR) MM is particularly challenging due to the heterogeneity of the disease, patient comorbidities, complications caused by the disease, and the adverse events (AEs) associated with individual treatments (Dimopoulos & Terpos, 2010; Laubach et al., 2011; Mohty et al., 2012; van de Donk et al., 2011).
Carfilzomib (Kyprolis) is a proteasome inhibitor that has shown safety and efficacy in patients with RR MM in both phase I (Alsina et al., 2012; O’Connor et al., 2009) and phase II trials (Badros et al., 2013; Jagannath et al., 2012; Siegel et al., 2012; Vij et al., 2012a, 2012b). Carfilzomib selectively and irreversibly inhibits the chymotrypsin-like activities of both the constitutive proteasome and the immunoproteasome via a binding mechanism that is distinct from that of the reversible inhibitor bortezomib (Velcade; Arastu-Kapur et al., 2011). Carfilzomib has a short half-life (Badros et al., 2013; O’Connor et al., 2009), which, along with its selective binding and lack of off-target effects, may explain the favorable safety profile of the drug.
Carfilzomib was recently approved by the US Food and Drug Administration (FDA) for patients with RR MM. Approval was based on results from the pivotal PX-171-003-A1 trial (ClinicalTrials.gov identifier NCT00511238) for efficacy and safety and studies PX-171-003-A0 (NCT00511238), PX-171-004 (NCT00530816), and PX-171-005 (NCT00721734) for safety (Onyx Pharmaceuticals, 2012; Siegel et al., 2012, 2013).
To maximize the benefits of carfilzomib, it is important to understand the possible AEs associated with its administration. The objective of this review is to summarize both common and infrequent clinically relevant AEs observed following carfilzomib use and to provide insight into the management of these events from an advanced practitioner (AP) perspective, based on clinical experience in the phase II trial setting as well as current use.
ADVERSE EVENTS IN CLINICAL TRIALS AND CLINICAL PRACTICE
A cross-trial safety analysis has been reported describing the safety and tolerability of single-agent carfilzomib in 526 patients with RR MM who received carfilzomib in four phase II trials: PX-171-003-A0, PX-171-003-A1, PX-171-004, and PX-171-005 (Harvey et al., 2012; Lonial, Niesvizky, McCulloch, Rajangam, & Vij, 2012; Martin et al., 2012; Nooka et al., 2012; Siegel et al., 2013). The most common hematologic AEs were anemia and thrombocytopenia, while the most common nonhematologic AEs were fatigue, nausea, dyspnea, diarrhea, pyrexia, and upper respiratory tract infections; see Table 1 (Onyx Pharmaceuticals, 2012; Siegel et al., 2013). The most common events ≥ grade 3 were mainly hematologic and included thrombocytopenia (23.4%), anemia (22.4%), lymphopenia (18.1%), pneumonia (10.5%), and neutropenia (10.3%). Of the 526 patients in this analysis, 14.6% required a dose modification and 14.8% discontinued treatment due to an AE (Siegel et al., 2013).
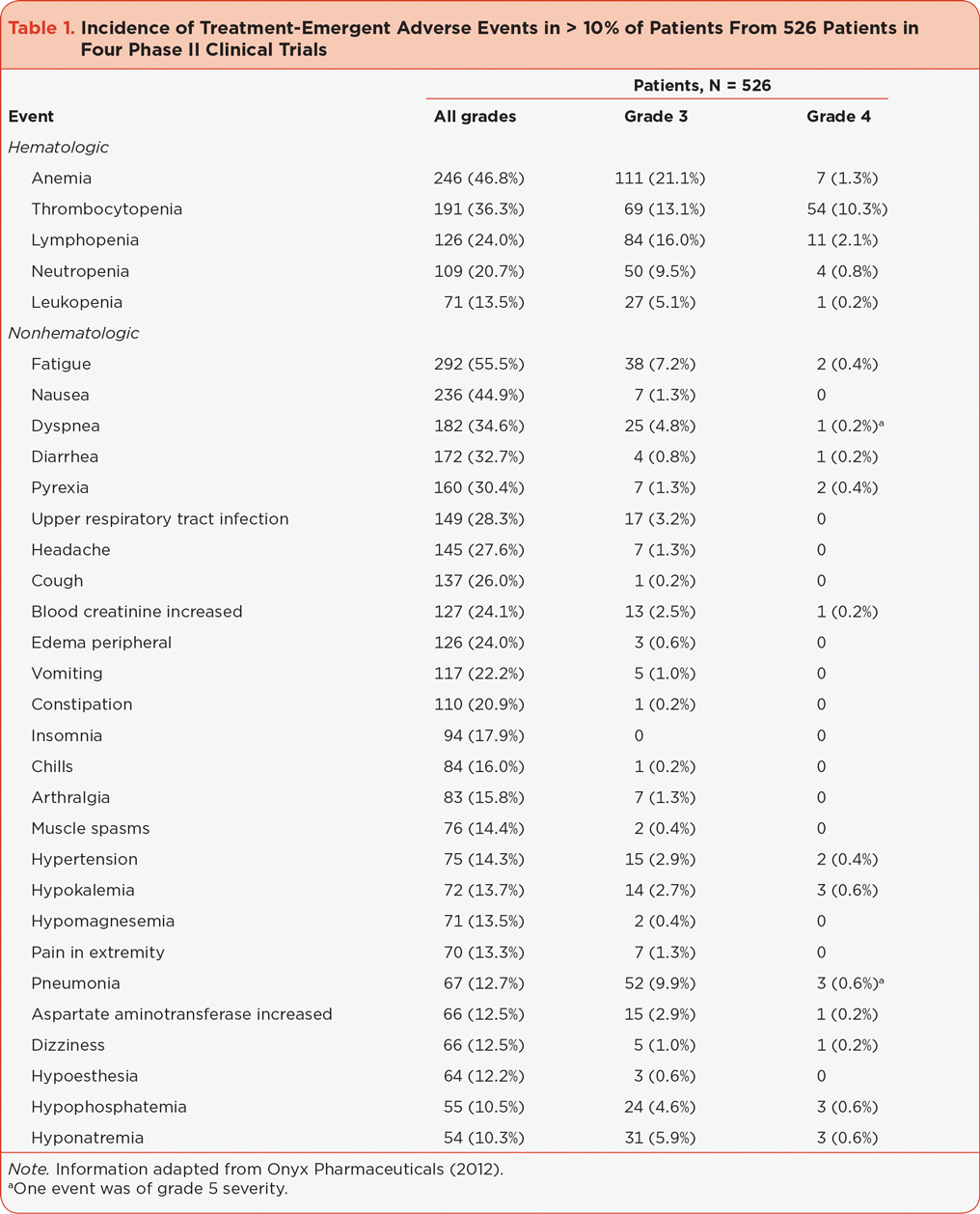
Adverse events frequently observed in the clinical setting can be found in Table 2, listed according to relative observed frequency; these events are generally consistent with those reported from the clinical trials noted above. Adverse events most commonly appearing in the first 24 to 48 hours include infusion-related reactions characterized by flu-like symptoms including fever, rigor, chills, and dyspnea; injection site–related reactions such as phlebitis; increases in certain laboratory values (including creatinine and transaminases); and elevations in blood pressure. Other common AEs that can appear either early or in later treatment cycles include fatigue, gastrointestinal AEs, hematologic AEs, dyspnea not related to initial dosing, infections, and similar increases in laboratory values described above. Cardiac, renal, and hepatic AEs are reported infrequently but can lead to serious complications.
RISK MANAGEMENT
Baseline Assessment
It is well-accepted that patient education is an important tool that can improve treatment. In order to obtain a thorough baseline profile of the patient, it is helpful to educate the patient on how to provide an adequate medical history. Particularly in individuals who are older and have been exposed to multiple therapies, such as those patients with RR MM, it is important to consider comorbid conditions and to have a thorough knowledge of the patient’s medical history. Performing a complete initial assessment is necessary to establish a baseline profile. In all patients with MM, baseline evaluation of cardiac and renal function is essential. Given that PN can be associated with treatment of MM (Mohty et al., 2010), a baseline neuropathy examination is important as well. However, neuropathy is not a significant concern with carfilzomib treatment (Martin et al., 2012). It is necessary to obtain baseline vital signs and monitor for changes during and after dosing, particularly in patients with a history of hypertension. A thorough review of previous and current medications is essential, especially for patients with a history of cardiovascular disease. During this initial evaluation, patients should be advised that concomitant medication records need to be updated frequently in order to properly anticipate and manage potential AEs. Additionally, a patient’s weight should be recorded at baseline and at least once weekly during treatment.
It is important to educate the patient on the need for prophylactic medication (which may vary according to the patient) to help manage AEs. It is also helpful to educate both patients and caregivers on the side effects that may occur with carfilzomib treatment, making sure they are aware of what should be reported and when to report it. Empowering patients and their caregivers with this knowledge will enable APs to manage patients who are taking carfilzomib more effectively.
Patient Management
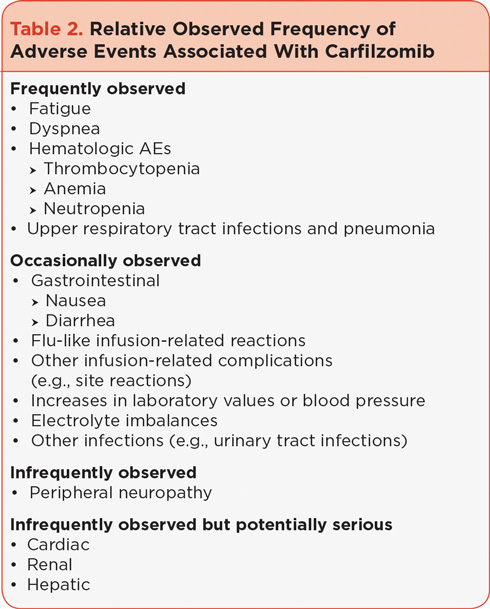
Many of the more frequently reported AEs such as fatigue, dyspnea, and infections can be alleviated with proper prophylactic measures, including hydration, dexamethasone, and antiviral/antibacterials (Table 3). Fatigue, which is commonly observed following carfilzomib use, was the most common treatment-emergent AE observed in the integrated safety analysis of several phase II clinical trials (Siegel et al., 2013). Dyspnea has been observed following carfilzomib treatment, both as part of flu-like infusion reactions (observed in early phase I studies [Alsina et al., 2012]) including fever, rigors, and chills, and at other times during treatment. Antiviral prophylaxis was used in the clinical trials to prevent viral infections in patients with MM, who typically have myelosuppression that can be attributed to their disease as well as their course of treatment (Dimopoulos & Terpos, 2010; Mateos, 2010).
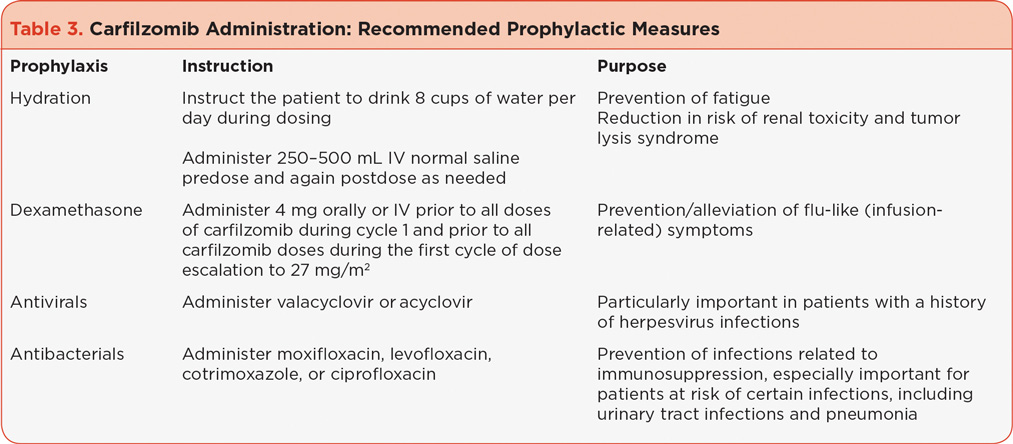
The carfilzomib prescribing information dosing guidelines recommend that patients receive 250 to 500 mL of normal saline or other appropriate intravenous (IV) fluid prior to the administration of carfilzomib and the same amount following administration, as needed (Onyx Pharmaceuticals, 2012). In clinical practice, following cycle 1, patients have been instructed to drink 8 cups of water prior to each dose of carfilzomib in order to prevent or reduce fatigue as well as to reduce the risk of other potential AEs, including renal toxicity and tumor lysis syndrome. It is, however, important to maintain homeostatic fluid balance; some patients may require a reduction in hydration or the addition of diuretics if they have signs or symptoms of fluid overload, including weight gain. It is also important to note that shortness of breath can be related to fluid retention. If hydration is needed post cycle 1 it can be reduced as necessary. Patients at high risk for fluid overload (e.g., those with preexisting congestive heart failure [CHF]) should be instructed to monitor their weight frequently after the first dose and report weight gains of 3 to 5 pounds above baseline to the clinical team.
Based on data from early clinical trials, administration of very low-dose dexamethasone (4 mg) prior to carfilzomib in cycles 1 and 2 is recommended to decrease the potential occurrence and severity of flu-like infusion-related symptoms, including dyspnea. In clinical practice, symptoms to watch for in the first 24 to 48 hours after each dose include fever, chills, myalgia, facial swelling or flushing, vomiting, weakness, shortness of breath, hypotension, and possible chest tightness (Table 4). Dexamethasone should be reintroduced if symptoms develop or reappear in subsequent cycles.
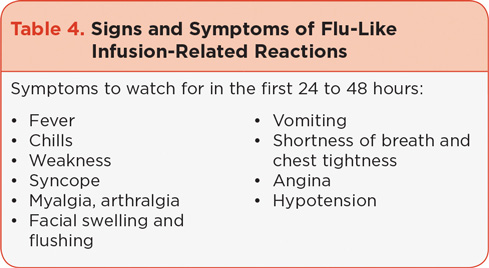
It is important for patients to be educated about the symptoms to watch out for. Additionally, caregivers should monitor the patient while he or she is answering a question to determine if they can speak without experiencing shortness of breath, which may occur after dosing. Shortness of breath at rest should also be closely monitored. The patient should be instructed to inform their health-care provider immediately if he or she experiences breathing difficulties so that any respiratory side effects can be managed appropriately, which may in turn decrease the likelihood of dose interruptions.
Antiviral prophylaxis (e.g., valacyclovir, acyclovir) is recommended for patients who have a history of herpes zoster infections (Onyx Pharmaceuticals, 2012). In the clinic, antiviral prophylaxis is often administered to all patients, and antibacterial prophylaxis should be considered for patients at risk of urinary tract infections, pneumonia, and other infections.
Managing Common Adverse Events
In the four phase II clinical trials that investigated carfilzomib, hematologic AEs related to myelosuppression were common but infrequently dose limiting (Nooka et al., 2012). In the 526 patients included in the phase II trials, ≥ grade 3 thrombocytopenia and neutropenia were reported in 23.4% and 10.3% of patients, respectively. Dosing guidelines recommend that the carfilzomib dose be withheld for grade 4 thrombocytopenia or ≥ grade 3 neutropenia (Onyx Pharmaceuticals, 2012).
Due to the nature of MM, thrombocytopenia is commonly seen in the clinic but is generally mild; discontinuation of therapy due to thrombocytopenia is low. Approximately a third of patients have > 50% plasmacytosis in their bone marrow at baseline, which leads to a reduction in patients’ platelet count prior to initiating carfilzomib. Platelet counts typically decrease during the dosing cycle and return to baseline prior to the next cycle. Thrombocytopenia is generally managed first with dose reduction and then with platelet transfusions if the platelet count falls below 25,000/ìL. The carfilzomib dose should be held for grade 4 thrombocytopenia until the platelet count surpasses 25,000/ìL. It is helpful to educate patients on the signs and symptoms of thrombocytopenia: unexpected bruising, petechiae, bleeding from the nose or gums, severe headaches, dizziness, and increased fatigue.
Grade 3 neutropenia is observed and commonly treated with growth factors including filgrastim (Neupogen), 300 or 480 ìg subcutaneously (SC) for 1 to 3 days followed by a complete blood count within a week, or pegfilgrastim (Neulasta) 6 mg SC per cycle. The carfilzomib dose is held for an absolute neutrophil count < 1000/ìL; generally patients are given growth factors and the carfilzomib dose is delayed for 1 to 2 days.
Anemia is usually grade 1 or 2 and managed with growth factors such as epoetin alfa (Procrit) 40,000 units SC weekly for hemoglobin ≤ 10 g/dL or darbepoetin alfa (Aranesp) 300 ìg SC every 2 weeks or 500 ìg each month for hemoglobin ≤ 10 mg/dL. Transfusions are rarely required for asymptomatic grade 1/2 anemia, and although growth factors can lead to side effects, the benefits of using them generally outweigh the risks. Anemia ≥ grade 3 (defined as hemoglobin < 8 g/dL, < 4.9 mmol/L [National Cancer Institute, 2010]) typically necessitates transfusions and sometimes requires dose interruptions and/or modifications. Rather than hold treatment because of anemia, carfilzomib can be administered followed by transfusion of packed cells as needed for hemoglobin < 8 mg/dL.
Gastrointestinal AEs including nausea, diarrhea, vomiting, and constipation were common in phase II studies; however, they were rarely dose limiting and rarely resulted in treatment discontinuation (Onyx Pharmaceuticals, 2012; Siegel et al., 2013). In clinical practice, these AEs are less worrisome as their frequency and severity vary significantly from patient to patient. Nausea is generally mild in severity and very manageable. Premedicating with antinausea medication is suggested including oral ondansetron 8 mg, 30 minutes prior to carfilzomib treatment. The majority of patients do not typically require additional nausea medication.
Hypomagnesemia and hypokalemia were observed in the four phase II studies and were mainly grades 1 or 2 (Onyx Pharmaceuticals, 2012). In the clinic, electrolyte imbalances that may require magnesium and potassium supplementation occur in some patients during the first cycle as well as throughout treatment. The imbalances are generally low grade and easily managed with oral supplements or increased dietary intake. There may be an occasional need for IV replacement with severe depletion. It is recommended that chemistry panels be checked at least once per cycle, with increased frequency in the first two cycles depending on the individual patient.
Consideration of Potential Adverse Events
It is important to comment on the AEs that occurred infrequently during the four phase II clinical trials but can potentially be serious nonetheless. Many of these AEs occur because of comorbidities that are observed in patients with MM: cardiac, renal, and hepatic AEs that can be exacerbated upon treatment.
Cardiac AEs have the potential to be serious; therefore, it is imperative to identify patients with confounding cardiac risk factors prior to initiating carfilzomib treatment. The integrated safety analysis of the phase II studies, from which patients with New York Heart Association Class III-IV heart failure or recent myocardial infarction/unstable angina were excluded, showed that > 70% of patients had a medical history of a cardiac event and 70% had cardiac risk factors at baseline, including hypertension, diabetes, hyperlipidemia, coronary artery disease, and CHF (Lonial et al., 2012). Overall, a dose reduction due to a cardiac AE was required in 6 patients (1.1%); 23 patients (4.4%) discontinued treatment due to a cardiac AE, including CHF (1.7%), arrhythmia (1.1%), and ischemic heart disease (1.0%; Lonial et al., 2012).
Renal dysfunction is common in patients with MM, and although a majority of the patients in the phase II carfilzomib studies had renal dysfunction at baseline, transient worsening of renal function was reported in 6% of patients. Nontransient worsening of renal function was reported in 7% of patients (Harvey et al., 2012). Serious hepatic disorders in the phase II trials were rare (Siegel et al., 2013).
In clinical practice, because of the presence of cardiac risk factors in many patients with RR MM prior to initiation of carfilzomib therapy, it is important to be aware of individual symptoms and concomitant medications and to manage them appropriately. In addition, a patient with cardiac risk factors should be cleared by a cardiologist prior to treatment. Medication adjustment may be required during carfilzomib treatment to manage blood pressure in hypertensive patients. For patients with CHF, baseline and repeat echocardiograms may be necessary, taking note of the ejection fraction as well as any signs of pulmonary hypertension. Patients with cardiac risk factors need to be closely monitored for fluid overload, which is most easily done by monitoring weight.
While renal impairment is not typically observed in response to carfilzomib treatment in clinical practice, it is important to recognize that small, typically transient increases in serum creatinine are often observed between the two consecutive doses. The increases are easily managed with adequate oral and IV hydration. Dose reduction is not normally required in patients with renal dysfunction, including patients on dialysis. Similar to renal impairment, hepatic impairment is not a significant issue in the clinic, although occasional elevations in transaminases are observed. In the event that hepatic enzymes are elevated, a review of the patient’s concomitant medications is suggested. The carfilzomib dose can be reduced if necessary.
Another AE of importance to patients with MM is PN, which is related either to the disease or to treatment and is frequently observed in patients with RR MM (Mohty et al., 2010). Peripheral neuropathy was not frequently reported during the four phase II clinical trials of carfilzomib even though most patients on study (71.9%) entered with a baseline of PN grade 1 or 2. Notably, newly developed PN occurred infrequently, and all reported cases of PN were generally ≤ grade 2 and rarely resulted in dose modifications or discontinuations (Martin et al., 2012). As in the clinical trial data summary, PN is rarely observed in the clinic.
CONCLUDING REMARKS
There are certain considerations that are important in the management of patients with MM treated with carfilzomib, and a recommended checklist to aid in patient care is provided in the Figure. Knowledge of the possible AEs associated with carfilzomib and the suggested guidelines for patient management prior to, during, and after treatment will help to prevent complications and ensure that prophylactic measures are implemented to reduce the frequency and severity of side effects. It is important to educate the patient on possible side effects and precautions and to explain the actions to be taken. Establishing a baseline profile and monitoring the patient closely throughout the course of treatment will help to personalize treatment while minimizing discomfort and AEs.

Finally, the care team should work together, sharing patient information and encouraging the patient to communicate any changes or side effects they experience. The overall treatment goal should be to maximize the efficacy of carfilzomib while minimizing AEs and discomfort in the patient. Being aware of the clinical practice experience with carfilzomib presented here should help APs to attain that goal.
ACKNOWLEDGMENT
The authors would like to thank Melissa Kirk, PhD (Fishawack Communications), for medical writing and editorial assistance, which was supported by Onyx Pharmaceuticals, Inc.
DISCLOSURE
Ms. McBride has received payment as a member of the Onyx Speakers Bureau and reimbursement for travel, accommodations, and meeting expenses from L&M Healthcare Communications. Ms. Samuel has received an honorarium as a member of the Carfilzomib Advisory Board.
REFERENCES
Alsina, M., Trudel, S., Furman, R. R., Rosen, P. J., O’Connor, O. A., Comenzo, R. L.,...Goy, A. (2012). A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clinical Cancer Research, 18(17), 4830–4840. http://dx.doi.org/10.1158/1078-0432.CCR-11-3007
American Cancer Society. (2013). Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society.
Arastu-Kapur, S., Anderl, J. L., Kraus, M., Parlati, F., Shenk, K. D., Lee, S. J.,...Kirk, C. J. (2011). Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: A link to clinical adverse events. Clinical Cancer Research, 17(9), 2734–2743. http://dx.doi.org/10.1158/1078-0432.CCR-10-1950
Badros, A. Z., Vij, R., Martin, T., Zonder, J. A., Kunkel, L., Wang, Z.,...Niesvizky, R. (2013). Carfilzomib in multiple myeloma patients with renal impairment: Pharmacokinetics and safety. Leukemia, 27, 1707–1714. http://dx.doi.org/10.1038/leu.2013.29
National Cancer Institute. (2010). Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. Bethesda, MD: National Cancer Institute. Retrieved from http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
Dimopoulos, M. A., & Terpos, E. (2010). Multiple myeloma. Annals of Oncology, 21(suppl 7), vii143–vii150. http://dx.doi.org/10.1093/annonc/mdq370
Harvey, R., Lonial, S., Patel, P., McCulloch, L., Niesvizky, R., & Kaufman, J. (2012). Carfilzomib dose and schedule need not be adjusted for baseline renal dysfunction, including patients on hemodialysis [Abstract 0844]. Haematologica, 97(suppl 1).
Jagannath, S., Vij, R., Stewart, A. K., Trudel, S., Jakubowiak, A. J., Reiman, T.,...Siegel, D. S. (2012). An open-label single-arm pilot phase II study (PX-171-003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. Clinical Lymphoma, Myeloma, & Leukemia, 12(5), 310–318. http://dx.doi.org/10.1016/j.clml.2012.08.003
Laubach, J., Mitsiades, M., CS, Mahindra, A., Luskin, M., Rosenblatt, J., Ghobrial, I.,...Richardson, P. (2011). Management of relapsed and relapsed/refractory multiple myeloma. Journal of the National Comprehensive Cancer Network, 9(10), 1209–1216.
Lonial, S., Niesvizky, R., McCulloch, L., Rajangam, K., & Vij, R. (2012). Cardiac and pulmonary safety profile of single-agent carfilzomib from four phase 2 studies in patients with relapsed and/or refractory multiple myeloma [Abstract 4037]. Blood (ASH Annual Meeting Abstracts), 120.
Ludwig, H., Beksac, M., Blade, J., Cavenagh, J., Cavo, M., Delforge, M.,...Palumbo, A. (2011). Multiple myeloma treatment strategies with novel agents in 2011: A European perspective. Oncologist, 16(4), 388–403. http://dx.doi.org/10.1634/theoncologist.2010-0386
Martin, T., Vij, R., Badros, A. Z., Patel, P., McCulloch, L., & Jagannath, S. (2012). Carfilzomib is associated with a low rate of typically mild to moderate, non-dose limiting treatment-emergent peripheral neuropathy [Abstract 0857]. Haematologica, 97(suppl 1).
Mateos, M. V. (2010). Management of treatment-related adverse events in patients with multiple myeloma. Cancer Treatment Reviews, 36(suppl 2), S24–S32. http://dx.doi.org/10.1016/S0305-7372(10)70009-8
Mohty, B., El-Cheikh, J., Yakoub-Agha, I., Avet-Loiseau, H., Moreau, P., & Mohty, M. (2012). Treatment strategies in relapsed and refractory multiple myeloma: A focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia, 26(1), 73–85. http://dx.doi.org/10.1038/leu.2011.310
Mohty, B., El-Cheikh, J., Yakoub-Agha, I., Moreau, P., Harousseau, J. L., & Mohty, M. (2010). Peripheral neuropathy and new treatments for multiple myeloma: Background and practical recommendations. Haematologica, 95(2), 311–319. http://dx.doi.org/10.3324/haematol.2009.012674
Nooka, A., Badros, A., Patel, P., McCulloch, L., Lonial, S., & Kaufman, J. (2012). Hematologic safety data from four phase II studies of single-agent carfilzomib in relapsed and/or refractory multiple myeloma [Abstract 8086]. Journal of Clinical Oncology (Meeting Abstracts), 30(15 suppl).
O’Connor, O. A., Stewart, A. K., Vallone, M., Molineaux, C. J., Kunkel, L. A., Gerecitano, J. F., & Orlowski, R. Z. (2009). A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clinical Cancer Research, 15(22), 7085–7091. http://dx.doi.org/10.1158/1078-0432.CCR-09-0822
Onyx Pharmaceuticals. (2012). Kyprolis (carfilzomib) package insert. Retrieved from http://www.kyprolis.com/Areas/Hcp/content/pdfs/PI.pdf
Pingali, S. R., Haddad, R. Y., & Saad, A. (2012). Current concepts of clinical management of multiple myeloma. Disease-a-Month, 58(4), 195–207. http://dx.doi.org/10.1016/j.disamonth.2012.01.006
Raab, M. S., Podar, K., Breitkreutz, I., Richardson, P. G., & Anderson, K. C. (2009). Multiple myeloma. Lancet, 374(9686), 324–339. http://dx.doi.org/10.1016/S0140-6736(09)60221-X
Siegel, D., Martin, T., Nooka, A., Harvey, R., Vij, R., Niesvizky, R.,...Lonial, S. (2013). Integrated safety profile of single-agent carfilzomib: Experience from 526 patients enrolled in 4 phase 2 clinical studies. Haematologica, 98 [E-pub ahead of print]. http://dx.doi.org/10.3324/haematol.2013.089334
Siegel, D., Martin, T., Wang, M., Vij, R., Jakubowiak, A., Lonial, S.,...Jagannath, S. (2012). A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood, 120(14), 2817–2825. http://dx.doi.org/10.1182/blood-2012-05-425934
van de Donk, N. W., Lokhorst, H. M., Dimopoulos, M., Cavo, M., Morgan, G., Einsele, H.,...Palumbo, A. (2011). Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treatment Reviews, 37(4), 266–283. http://dx.doi.org/10.1016/j.ctrv.2010.08.008
Vij, R., Siegel, D., Jagannath, S., Jakubowiak, A., Stewart, A., McDonagh, K.,...Wang, M. (2012a). An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. British Journal of Haematology, 158(6), 739–748. http://dx.doi.org/10.1111/j.1365-2141.2012.09232.x
Vij, R., Wang, M., Kaufman, J. L., Lonial, S., Jakubowiak, A. J., Stewart, A. K.,...Siegel, D. S. (2012b). An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood, 119(24), 5661–5670. http://dx.doi.org/10.1182/blood-2012-03-414359