Carfilzomib
From Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania
Dr. Vozniak is an advisory board member for Onyx Pharmaceuticals, Inc.
Correspondence to: J. Michael Vozniak, PharmD, BCOP, Hospital of the University of Pennsylvania, Department of Pharmacy, Silverstein Building, Philadelphia, PA 19104. E-mail: michael.vozniak@uphs.upenn.edu
J Adv Pract Oncol 2013;4(Suppl 1):15–21 | © 2013 Harborside Press®


ARTICLE
Multiple myeloma (MM), a B-cell malignancy in which there is an expansion and accumulation of abnormal plasma cells in the bone marrow (Dimopoulos & Terpos, 2010), accounts for 10% of all hematologic cancers. In 2013, an estimated 22,350 new cases of MM will be diagnosed and 10,710 deaths are expected in the United States (American Cancer Society [ACS], 2013; Siegel, Naishadham, & Jemal, 2012). Characteristic symptoms of MM include calcium elevation, renal insufficiency, anemia, and lytic bone lesions, commonly referred to by the mnemonic CRAB (Dimopoulos & Terpos, 2010; Raab, Podar, Breitkreutz, Richardson, & Anderson, 2009).
The survival rate for patients diagnosed with MM has significantly improved over the past decade with the development of newer treatments, but MM remains incurable (Eshaghian & Berenson, 2012). Patients with relapsed disease—especially those who have become refractory to available treatments—have a poor prognosis, with median event-free survival of 5 months and overall survival of 9 months (Kumar et al., 2012). Treatment and management of patients with relapsed and refractory (RR) MM is complicated by the symptoms of the disease as well as side effects associated with the treatments (Laubach et al., 2011; van de Donk et al., 2011).
Proteasome inhibition is an effective treatment approach for MM (McBride & Ryan, 2013). Two proteasome inhibitors approved by the US Food and Drug Administration (FDA) for the treatment of MM are bortezomib (Velcade) and carfilzomib (Kyprolis). Bortezomib, a dipeptidyl boronic acid derivative proteasome inhibitor, was approved for the treatment of MM nearly 10 years ago (Rajkumar, Richardson, Hideshima, & Anderson, 2005). Carfilzomib is a selective proteasome inhibitor that was granted FDA approval in 2012 for the treatment of patients with MM who have received at least two prior therapies, including bortezomib and an immunomodulatory agent, and who have demonstrated disease progression on or within 60 days of the last treatment (Onyx Pharmaceuticals, 2012).
PHARMACOLOGY
Mechanism of Action
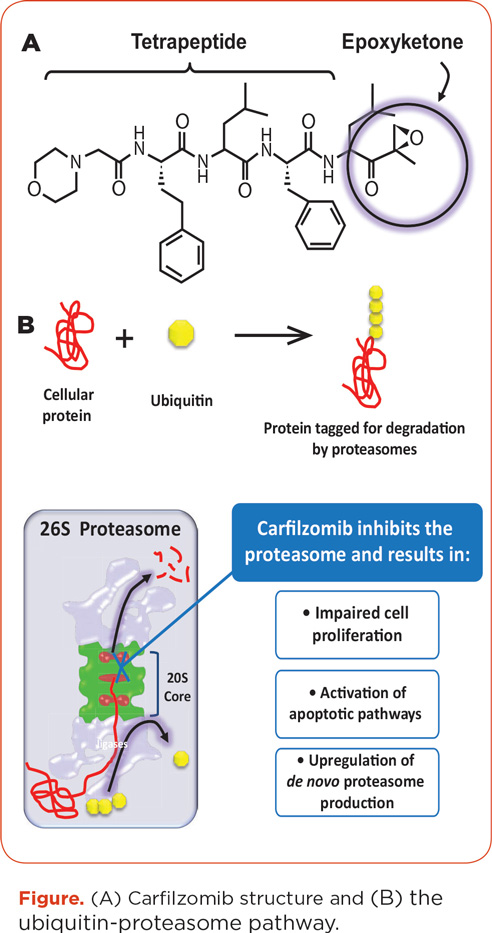
Proteasomes, which are present in all eukaryotic cells, degrade ubiquitinated proteins and thus influence cellular processes, including proliferation and DNA repair (Kortuem & Stewart, 2013). Proteasome inhibition can also induce cell cycle arrest and apoptosis by inducing an unfolded protein stress response. The constitutive 26S proteasome carries three catalytic sites that can be inhibited by proteasome inhibitors: the chymotrypsin, trypsin, and caspase-like sites. Additionally, immunoproteasomes exist in immune or hematopoietic cells. Multiple myeloma cells are particularly susceptible to proteasome inhibitors due to their inherent function in antibody production (Kortuem & Stewart, 2013). Carfilzomib and bortezomib both inhibit constitutive proteasomes and immunoproteasomes present in MM cells (Kuhn, Orlowski, & Bjorklund, 2011).
Carfilzomib, an epoxyketone tetrapeptide proteasome inhibitor (see Figure), is an analog of epoxomycin, a natural proteasome inhibitor shown to specifically inhibit the chymotrypsin-like (CT-L) activity of the 20S proteasome core (Kuhn et al., 2007; Meng et al., 1999). Carfilzomib is structurally and mechanistically distinct from bortezomib: The latter forms a reversible bond with the proteasome (Arastu-Kapur et al., 2011; Kortuem & Stewart, 2013), while carfilzomib forms an irreversible, covalent bond with a catalytic site of the proteasome and an analogous site of the immunoproteasome (Demo et al., 2007). Additionally, in preclinical models, carfilzomib showed dose- and time-dependent inhibition of proliferation, was more selective than bortezomib at therapeutic concentrations, and showed little activity against the trypsin-like or caspase-like activities of the proteasome (Demo et al., 2007; Kuhn et al., 2007). Moreover, carfilzomib showed significantly less preclinical neurotoxicity and neurodegeneration than bortezomib (Arastu-Kapur et al., 2011). The specificity and irreversible binding of carfilzomib may give it a tolerability advantage as well as an efficacy advantage over bortezomib (Arastu-Kapur et al., 2011; Yang et al., 2011).
Pharmacokinetics/Pharmacodynamics
Carfilzomib is extensively metabolized into inactive metabolites (Yang et al., 2011) and has a short half-life, with the majority of drug eliminated from plasma within 30 minutes (Alsina et al., 2012; Badros et al., 2013; O’Connor et al., 2009). Carfilzomib is distributed to all tissues except the brain (Demo et al., 2007) and is largely metabolized extrahepatically, with < 1% excreted intact (Yang et al., 2011). The rapid clearance of carfilzomib may contribute to the tolerability of the drug. A drug interaction study showed that cytochrome P450 pathways play only a minor role in carfilzomib metabolism (Wang et al., 2013). Clinical studies to date have not demonstrated any need for avoidance of any concomitant medications due to drug-drug interactions (Onyx Pharmaceuticals, 2012). Both the area under the curve and maximum concentration of carfilzomib increased with increasing carfilzomib doses, but the increases were not dose-dependent (Alsina et al., 2012; O’Connor et al., 2009). Additionally, renal insufficiency does not appear to affect the pharmacokinetics of carfilzomib (Badros et al., 2013).
In pharmacodynamic studies of carfilzomib, the CT-L activity of the proteasome and the immunoproteasome were 70% to 80% inhibited at lower doses and approximately 90% inhibited after a dose of 27 mg/m2; progressive recovery of proteasome activity occurred at 24 and 72 hours, and complete recovery was observed on day 1, cycle 2, following a 12-day rest period (Alsina et al., 2012; Arastu-Kapur et al., 2011; Demo et al., 2007; Kuhn et al., 2007; O’Connor et al., 2009).
DOSING AND ADMINISTRATION
Carfilzomib dose is calculated based on the patient’s body surface area at baseline (treatment initiation). Patients with a body surface area of > 2.2 m2 should receive a dose based on a body surface area of 2.2 m2 (Onyx Pharmaceuticals, 2012). Dose adjustments are only necessary for weight changes > 20%. Carfilzomib is administered at a dose of 20 mg/m2 in cycle 1 and, if tolerated, should be increased to 27 mg/m2 beginning in cycle 2 and continuing in subsequent cycles. Carfilzomib is supplied in single-use vials containing 60 mg sterile lyophilized powder, which should be reconstituted with 29 mL sterile water (Onyx Pharmaceuticals, 2012). Carfilzomib is administered intravenously (IV) over 2 to 10 minutes on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle. The IV catheter should be flushed with 0.9% saline or 5% dextrose solution immediately before and after administration. Carfilzomib should not be administered as a bolus.
It is recommended that patients receive proper prophylaxis prior to carfilzomib treatment. Immediately prior to carfilzomib administration, patients should receive 250 to 500 mL of normal saline or other appropriate IV fluid and should receive the same amount following administration as needed to reduce the risk of fatigue and other potential adverse events (AEs), including tumor lysis syndrome and renal toxicities (Onyx Pharmaceuticals, 2012). Additionally, prophylaxis with dexamethasone 4 mg prior to all doses in cycle 1 and during the first cycle of dose escalation to 27 mg/m2 is recommended to help reduce the risk of potential flu-like infusion-related symptoms, including dyspnea. Dexamethasone premedication can be reinstated in later cycles if symptoms develop or reappear.
KEY CLINICAL STUDIES
In phase I trials and a pilot phase II trial, carfilzomib showed efficacy in patients with RR MM. The phase I trials showed efficacy both with doses ranging from 1.2 to 20 mg/m2 on days 1 through 5 of a 14-day cycle (PX-171-001 study) and in a dose-escalation study (PX-171-002) using the now approved dosing schedule (O’Connor et al., 2009; Alsina et al., 2012). In the latter study, 4 of the 9 patients who started carfilzomib at ≥ 20 mg/m2 had a partial response (PR). In 42 response-evaluable patients in a pilot phase II study in which carfilzomib was administered at 20 mg/m2 (PX-171-003-A0), overall response rate (ORR, ≥ PR) was 16.7% and the median duration of response (DOR) was 7.2 months (Jagannath et al., 2012).
Accelerated approval of carfilzomib was based on the efficacy observed in the PX-171-003-A1 phase II study (003-A1) of 266 patients with RR MM in which the current recommended dose and schedule were established (Siegel et al., 2012). The ORR was 23.7%, progression-free survival (PFS) was 3.7 months, DOR was 7.8 months in the 61 patients who achieved ≥ PR, and overall survival (OS) was 15.4 months.
In the PX-171-004 phase II study, which had a very similar patient population as 003-A1, patients were stratified according to their prior bortezomib treatment status. In patients who had prior treatment with bortezomib, ORR was 17.1%, DOR was > 10.6 months, and the median time to progression (TTP) was 4.6 months (Vij et al., 2012a). Predictably, the responses were better in bortezomib-naive patients, who were separated into cohorts that received carfilzomib 20 mg/m2 (cohort 1) and carfilzomib 20/27 mg/m2 (cohort 2; Vij et al., 2012b). In bortezomib-naive patients, ORR was 47.6% (42.4% in cohort 1 and 52.2% in cohort 2) and PFS was 8.2 and > 11.5 months, respectively. Duration of response was 13.1 months for cohort 1 (not reached for cohort 2), and TTP was 12.0 months in both cohorts combined.
In a fourth phase II study, patients with varying degrees of renal impairment (including those on dialysis) were evaluated for ORR. Thirty-six patients with renal impairment had an ORR of 27.7% compared with an ORR of 25.5% in the overall group (N = 50; Badros et al., 2013). The subset of 11 patients without renal insufficiency had an ORR of 18.2%. In this study, there were no appreciable differences in carfilzomib clearance or exposure among the patients, regardless of renal function.
ADVERSE EFFECTS
Safety data for single-agent carfilzomib have been analyzed and summarized in a cross-study analysis of 526 patients with advanced MM who took part in four phase II trials: 003-A0, 003-A1, 004, and 005. In this analysis, 14.6% of patients required a dose modification or reduction, and 14.8% discontinued treatment due to an AE (Siegel et al., 2013). A summary of the most common AEs occurring in > 20% of patients receiving carfilzomib is presented in Table 1. The greatest proportion of AEs were hematologic in nature, and nonhematologic AEs were mainly grade 1 or 2 (Onyx Pharmaceuticals, 2012). In the cross-study analysis, specific groups of AEs were analyzed in detail, including hematologic, cardiac, and renal effects as well as peripheral neuropathy (PN).

Upon study entry, any grade hematologic AEs were reported in patients as follows: 37.4% thrombocytopenia, 65.4% lymphopenia, 64.4% neutropenia, and 89.3% anemia. Hematologic AEs were common in these studies, including anemia in 46.8% of patients and thrombocytopenia in 37.8% of patients, but were generally transient and not dose-limiting. Among thrombocytopenia, lymphopenia, neutropenia, and anemia AEs, ≤ 1.1% of patients required a dose reduction and ≤ 1.0% discontinued treatment for each (Nooka et al., 2012).
Overall, 73.6% of patients had a history of cardiovascular events; patients fit this category if they were taking ≥ 1 cardiac medication at study entry. The most common grouped cardiac AEs were cardiac arrhythmia (13.3%), cardiac failure (7.2%), and ischemic heart disease (3.4%). Dose reductions and discontinuations due to a cardiac AE were low at 1.1% and 4.4%, respectively. There were 8 (1.5%) cardiac-related deaths (all related to carfilzomib): 5 patients (1.0%) died due to a cardiac AE and an additional 3 patients had a cardiac component to their death (Lonial, Niesvisky, McCulloch, Rajangam, & Vij, 2012).
At baseline, 23.8% of patients had moderate to severe renal dysfunction (creatinine clearance [CrCl] < 50 mL/min), and 39.4% had mild renal dysfunction (CrCl ≥ 50 to < 80 mL/min). Overall, 33.1% of patients had ≥ 1 renal AE (78.2% of which were grade 1 or 2), including increased blood creatinine (24.1%), acute renal failure (ARF, 5.3%), renal failure (3.8%), and increased blood urea (2.7%). The two deaths attributed to renal AEs were ARF due to septic shock and a grade 5 event of “progressive renal failure,” both unrelated to carfilzomib. Overall, 21 patients (4.0%) discontinued treatment due to a renal AE, and 19 (10.9%) required a dose reduction. Fifty percent of patients with an ARF AE did not require a change in carfilzomib therapy (Harvey et al., 2012).
Most patients (84.8%) had a history of PN with prior treatments, including bortezomib (42.6%), thalidomide (43.3%), and lenalidomide (5.9%). During treatment with carfilzomib, overall PN was 13.9% (7.8% grade 1, 4.8% grade 2, 1.3% grade 3, and none ≥ grade 4). Although 71.9% of patients had active PN at baseline, the majority (87.3% [330/378]) did not report AEs related to PN while receiving carfilzomib. In response to a PN AE, 1 patient (0.2%) discontinued treatment, and 4 (0.8%) required a dose reduction (Martin et al., 2012).
ROLE IN THERAPY
Proteasome inhibitors have been used to treat patients with RR MM for nearly 10 years; they are listed among the current National Comprehensive Cancer Network (NCCN) recommendations for MM therapy (category 1 for bortezomib), both as single-agent salvage therapy for progressive disease and in combination for primary therapy (NCCN, 2013). The NCCN’s MM Panel members classified salvage regimen options for progressive MM as either “preferred regimens” or “other regimens.” The panel classified carfilzomib as a preferred regimen based on the phase II 003-A1 study results (Anderson et al., 2013). Single-agent carfilzomib is recommended for use in patients with progressive MM who have had at least two prior therapies, including bortezomib and an immunomodulatory agent.
The results of ongoing carfilzomib studies, particularly the phase III trials, will provide insight into the optimal use of carfilzomib for all patients. In these trials, direct comparisons are being made between carfilzomib plus low-dose dexamethasone and bortezomib plus low-dose dexamethasone (ENDEAVOR, ClinicalTrials.gov identifier NTC01568866) and for lenalidomide plus low-dose dexamethasone with or without added carfilzomib (ASPIRE, NCT01080391). The FOCUS trial is evaluating single-agent carfilzomib vs. best supportive care with an endpoint of OS (NCT01302392; Hájek, Bryce, Ro, Klencke, & Ludwig, 2012). Additionally, a phase I/II trial of carfilzomib combined with lenalidomide and low-dose dexamethasone has shown encouraging results in patients with newly diagnosed MM (Jakubowiak et al., 2013), as well as a phase III multicenter, open-label, randomized study in transplant-ineligible patients with newly diagnosed MM (CLARION, NCT01818752).
IMPLICATIONS
Carfilzomib has a tolerable safety profile and is a promising treatment option in patients with RR MM. Patients being considered for treatment should have a thorough initial assessment to provide an accurate baseline profile. In particular, a complete cardiac assessment should be performed in any patients with cardiac risk factors. Complete baseline vital signs and laboratory values are also important so that changes can be closely monitored. Patients with preexisting PN may be treated with carfilzomib, as it generally does not exacerbate PN. Patients should receive pre- and postdose hydration along with dexamethasone premedication prior to each cycle 1 dose and with the first dose of 27 mg/m2 and in subsequent cycles as needed.
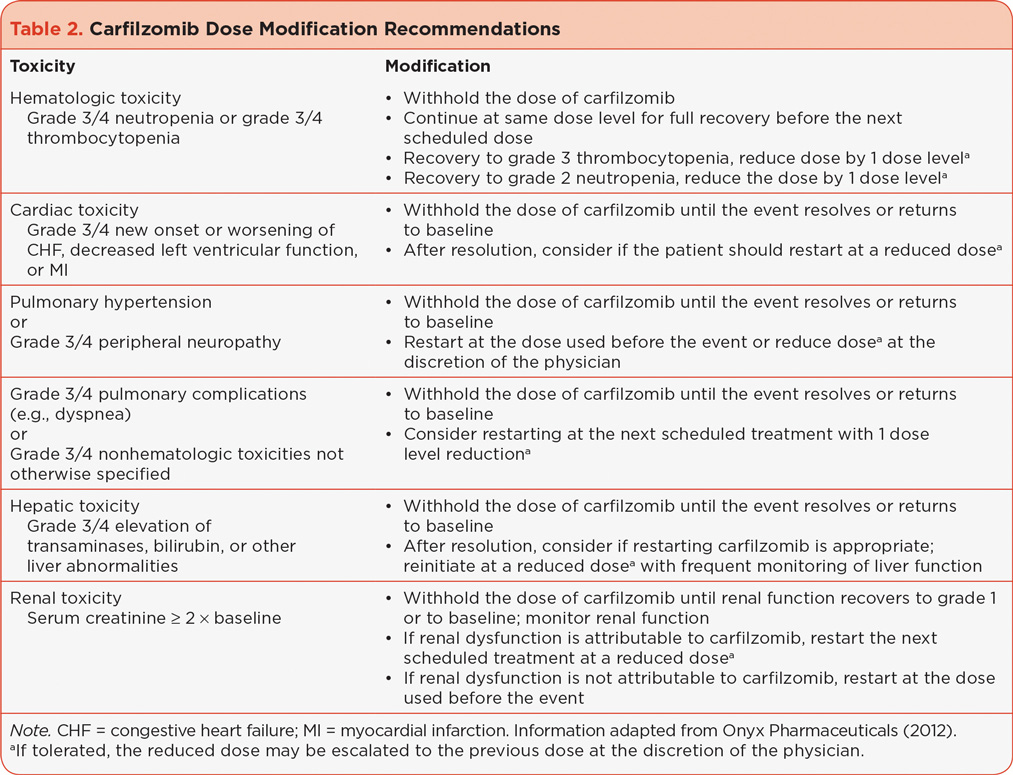
Dosing modification recommendations have been established for carfilzomib and are summarized in Table 2 (Onyx Pharmaceuticals, 2012). Appropriate and frequent monitoring is important to detect AEs that may affect carfilzomib dosing. With adequate monitoring, it is generally possible to keep patients on effective carfilzomib doses, as observed with the low rates of dose modifications and discontinuations in the cross-trial safety analysis (Onyx Pharmaceuticals, 2012). The ultimate treatment goal for patients with RR MM is to be able to stay on effective carfilzomib doses while minimizing AEs and patient discomfort.
SUMMARY
Carfilzomib is a well-tolerated therapy that has demonstrated encouraging efficacy in patients with RR MM. Because of its tolerable side effects, minimal dose modifications or interruptions are required, which allows patients with RR MM to achieve maximum benefit. Adequate prophylaxis with hydration and dexamethasone is required. Additionally, routine patient monitoring and AE management are important to maintain effective carfilzomib dosing.
ACKNOWLEDGMENTS
The authors would like to thank Melissa Kirk, PhD (Fishawack Communications), for medical writing and editorial assistance, which was supported by Onyx Pharmaceuticals, Inc.
DISCLOSURE
Dr. Vozniak is an advisory board member for Onyx Pharmaceuticals, Inc.
REFERENCES
Alsina, M., Trudel, S., Furman, R. R., Rosen, P. J., O’Connor, O. A., Comenzo, R. L.,...Goy, A. (2012). A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clinical Cancer Research, 18(17), 4830–4840. http://dx.doi.org/10.1158/1078-0432.CCR-11-3007
American Cancer Society. (2013). Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society.
Anderson, K. C., Alsina, M., Bensinger, W., Biermann, J. S., Cohen, A. D., Devine, S.,...Kumar, S. (2013). NCCN clinical practice guidelines in oncology: Multiple myeloma. Journal of the National Comprehensive Cancer Network, 11(1), 11–17.
Arastu-Kapur, S., Anderl, J. L., Kraus, M., Parlati, F., Shenk, K. D., Lee, S. J.,...Kirk, C. J. (2011). Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: A link to clinical adverse events. Clinical Cancer Research, 17(9), 2734–2743. http://dx.doi.org/10.1158/1078-0432.CCR-10-1950
Badros, A. Z., Vij, R., Martin, T., Zonder, J. A., Kunkel, L., Wang, Z.,...Niesvizky, R. (2013). Carfilzomib in multiple myeloma patients with renal impairment: Pharmacokinetics and safety. Leukemia, 27, 1707–1714. http://dx.doi.org/10.1038/leu.2013.29
Demo, S. D., Kirk, C. J., Aujay, M. A., Buchholz, T. J., Dajee, M., Ho, M. N.,...Bennett, M. K. (2007). Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Research, 67(13), 6383–6391. http://dx.doi.org/10.1158/0008-5472.CAN-06-4086
Dimopoulos, M. A., & Terpos, E. (2010). Multiple myeloma. Annals of Oncology, 21(suppl 7), vii143–vii150. http://dx.doi.org/10.1093/annonc/mdq370
Eshaghian, S., & Berenson, J. R. (2012). Multiple myeloma: Improved outcomes with new therapeutic approaches. Current Opinion in Supportive and Palliative Care, 6(3), 330–336. http://dx.doi.org/10.1097/SPC.0b013e3283565c56
Hájek, R., Bryce, R., Ro, S., Klencke, B., & Ludwig, H. (2012). Design and rationale of FOCUS (PX-171-011): A randomized, open-label, phase 3 study of carfilzomib vs. best supportive care regimen in patients with relapsed and refractory multiple myeloma (R/R MM). BMC Cancer, 12, 415. http://dx.doi.org/10.1186/1471-2407-12-415
Harvey, R., Lonial, S., Patel, P., McCulloch, L., Niesvizky, R., & Kaufman, J. (2012). Carfilzomib dose and schedule need not be adjusted for baseline renal dysfunction, including patients on hemodialysis [Abstract 0844]. Haematologica, 97(suppl 1).
Jagannath, S., Vij, R., Stewart, A. K., Trudel, S., Jakubowiak, A. J., Reiman, T.,...Siegel, D. S. (2012). An open-label single-arm pilot phase II study (PX-171-003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. Clinical Lymphoma, Myeloma, & Leukemia, 12(5), 310–318. http://dx.doi.org/10.1016/j.clml.2012.08.003
Jakubowiak, A., Dytfeld, D., Jasielec, J., Griffith, K., Lebovic, D., Vesole, D.,...Vij, R. (2013). Carfilzomib, lenalidomide, low-dose dexamethasone (CRd) in elderly patients with newly diagnosed multiple myeloma (NDMM) [Abstract O-10]. Clinical Lymphoma, Myeloma, & Leukemia, 13(suppl 1).
Kortuem, K. M., & Stewart, A. K. (2013). Carfilzomib. Blood, 121(6), 893–897. http://dx.doi.org/10.1182/blood-2012-10-459883
Kuhn, D. J., Chen, Q., Voorhees, P. M., Strader, J. S., Shenk, K. D., Sun, C. M.,...Orlowski, R. Z. (2007). Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood, 110(9), 3281–3290. http://dx.doi.org/10.1182/blood-2007-01-065888
Kuhn, D. J., Orlowski, R. Z., & Bjorklund, C. C. (2011). Second generation proteasome inhibitors: Carfilzomib and immunoproteasome-specific inhibitors (IPSIs). Current Cancer Drug Targets, 11(3), 285–295.
Kumar, S. K., Lee, J. H., Lahuerta, J. J., Morgan, G., Richardson, P. G., Crowley, J.,...Durie, B. G. (2012). Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter International Myeloma Working Group study. Leukemia, 26(1), 149–157. http://dx.doi.org/10.1038/leu.2011.196
Laubach, J., Mitsiades, M., CS, Mahindra, A., Luskin, M., Rosenblatt, J., Ghobrial, I.,...Richardson, P. (2011). Management of relapsed and relapsed/refractory multiple myeloma. Journal of the National Comprehensive Cancer Network, 9(10), 1209–1216.
Lonial, S., Niesvizky, R., McCulloch, L., Rajangam, K., & Vij, R. (2012). Cardiac and pulmonary safety profile of single-agent carfilzomib from four phase 2 studies in patients with relapsed and/or refractory multiple myeloma [Abstract 4037]. Blood (ASH Annual Meeting Abstracts), 120.
Martin, T., Vij, R., Badros, A. Z., Patel, P., McCulloch, L., & Jagannath, S. (2012). Carfilzomib is associated with a low rate of typically mild to moderate, non-dose limiting treatment-emergent peripheral neuropathy [Abstract 0857]. Haematologica, 97(suppl 1).
McBride, A., & Ryan, P. Y. (2013). Proteasome inhibitors in the treatment of multiple myeloma. Expert Review of Anticancer Therapy, 13(3), 339–358. http://dx.doi.org/10.1586/era.13.9
Meng, L., Mohan, R., Kwok, B. H., Elofsson, M., Sin, N., & Crews, C. M. (1999). Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proceedings of the National Academy of Sciences of the USA, 96(18), 10403–10408.
National Comprehensive Cancer Network. (2013). NCCN Clinical Practice Guidelines in Oncology: Multiple myeloma, version 1.2013.
Nooka, A., Badros, A., Patel, P., McCulloch, L., Lonial, S., & Kaufman, J. (2012). Hematologic safety data from four phase II studies of single-agent carfilzomib in relapsed and/or refractory multiple myeloma [Abstract 8086]. Journal of Clinical Oncology (Meeting Abstracts), 30(15 suppl).
O’Connor, O. A., Stewart, A. K., Vallone, M., Molineaux, C. J., Kunkel, L. A., Gerecitano, J. F., & Orlowski, R. Z. (2009). A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clinical Cancer Research, 15(22), 7085–7091. http://dx.doi.org/10.1158/1078-0432.CCR-09-0822
Onyx Pharmaceuticals. (2012). Kyprolis (carfilzomib) package insert. Retrieved from http://www.kyprolis.com/Areas/Hcp/content/pdfs/PI.pdf
Raab, M. S., Podar, K., Breitkreutz, I., Richardson, P. G., & Anderson, K. C. (2009). Multiple myeloma. Lancet, 374(9686), 324–339. http://dx.doi.org/10.1016/S0140-6736(09)60221-X
Rajkumar, S. V., Richardson, P. G., Hideshima, T., & Anderson, K. C. (2005). Proteasome inhibition as a novel therapeutic target in human cancer. Journal of Clinical Oncology, 23(3), 630–639. http://dx.doi.org/10.1200/JCO.2005.11.030
Siegel, D., Martin, T., Nooka, A., Harvey, R., Vij, R., Niesvizky, R.,...Lonial, S. (2013). Integrated safety profile of single-agent carfilzomib: Experience from 526 patients enrolled in 4 phase 2 clinical studies. Haematologica, 98 [E-pub ahead of print]. http://dx.doi.org/10.3324/haematol.2013.089334
Siegel, D., Martin, T., Wang, M., Vij, R., Jakubowiak, A., Lonial, S.,...Jagannath, S. (2012). A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood, 120(14), 2817–2825. http://dx.doi.org/10.1182/blood-2012-05-425934
Siegel, R., Naishadham, D., & Jemal, A. (2012). Cancer statistics, 2012. CA Cancer J Clin, 62(1), 10–29. http://dx.doi.org/10.3322/caac.20138
van de Donk, N. W., Lokhorst, H. M., Dimopoulos, M., Cavo, M., Morgan, G., Einsele, H.,...Palumbo, A. (2011). Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treatment Reviews, 37(4), 266–283. http://dx.doi.org/10.1016/j.ctrv.2010.08.008
Vij, R., Siegel, D., Jagannath, S., Jakubowiak, A., Stewart, A., McDonagh, K.,...Wang, M. (2012a). An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. British Journal of Haematology, 158(6), 739–748. http://dx.doi.org/10.1111/j.1365-2141.2012.09232.x
Vij, R., Wang, M., Kaufman, J. L., Lonial, S., Jakubowiak, A. J., Stewart, A. K.,...Siegel, D. S. (2012b). An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood, 119(24), 5661–5670. http://dx.doi.org/10.1182/blood-2012-03-414359
Wang, Z., Yang, J., Kirk, C., Fang, Y., Alsina, M., Badros, A.,...Infante, J. R. (2013). Clinical pharmacokinetics, metabolism, and drug-drug interaction of carfilzomib. Drug Metabolism and Disposition, 41(1), 230–237. http://dx.doi.org/10.1124/dmd.112.047662
Yang, J., Wang, Z., Fang, Y., Jiang, J., Zhao, F., Wong, H.,...Kirk, C. J. (2011). Pharmacokinetics, pharmacodynamics, metabolism, distribution, and excretion of carfilzomib in rats. Drug Metabolism and Disposition, 39(10), 1873–1882. http://dx.doi.org/10.1124/dmd.111.039164